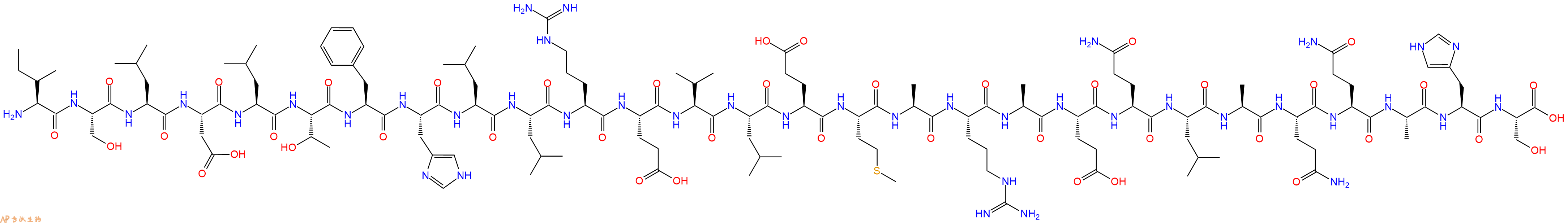
CRF(6-33)(human) TFA 是一种 CRF 结合蛋白配体 (CRF-BP) 抑制剂,与 CRF-BP 竞争性结合,但不与突触后 CRF 受体结合。CRF(6-33)(human) TFA 具有抗肥胖作用。
编号:117224
CAS号:120066-38-8
单字母:H2N-ISLDLTFHLLREVLEMARAEQLAQQAHS-OH
| 编号: | 117224 |
| 中文名称: | CRF(6-33)(human) TFA |
| 英文名: | CRF(6-33)(human) TFA |
| CAS号: | 120066-38-8 |
| 单字母: | H2N-ISLDLTFHLLREVLEMARAEQLAQQAHS-OH |
| 三字母: | H2N N端氨基 -Ile异亮氨酸 -Ser丝氨酸 -Leu亮氨酸 -Asp天冬氨酸 -Leu亮氨酸 -Thr苏氨酸 -Phe苯丙氨酸 -His组氨酸 -Leu亮氨酸 -Leu亮氨酸 -Arg精氨酸 -Glu谷氨酸 -Val缬氨酸 -Leu亮氨酸 -Glu谷氨酸 -Met甲硫氨酸 -Ala丙氨酸 -Arg精氨酸 -Ala丙氨酸 -Glu谷氨酸 -Gln谷氨酰胺 -Leu亮氨酸 -Ala丙氨酸 -Gln谷氨酰胺 -Gln谷氨酰胺 -Ala丙氨酸 -His组氨酸 -Ser丝氨酸 -OHC端羧基 |
| 氨基酸个数: | 28 |
| 分子式: | C141H231N41O43S1 |
| 平均分子量: | 3220.66 |
| 精确分子量: | 3218.69 |
| 等电点(PI): | 6.51 |
| pH=7.0时的净电荷数: | -0.55 |
| 平均亲水性: | -0.075 |
| 疏水性值: | 0.04 |
| 外观与性状: | 白色粉末状固体 |
| 消光系数: | - |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 纯度: | 95%、98% |
| 盐体系: | 可选TFA、HAc、HCl或其它 |
| 生成周期: | 2-3周 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 抑制剂相关肽(Inhibitor Peptide) ACTH及相关肽 |
CRF(6-33)(human) TFA 是一种 CRF 结合蛋白配体 (CRF-BP) 抑制剂,与 CRF-BP 竞争性结合,但不与突触后 CRF 受体结合。CRF(6-33)(human) TFA 具有抗肥胖作用。
CRF(6-33)(human) TFA is a CRF binding protein (CRF-BP) ligand inhibitor. CRF(6-33)(human) TFA competitively binds the CRF-BP but not the post-synaptic CRF receptors. CRF(6-33)(human) TFA has anti-obesity effect.
CRF(6-33)醋酸盐被发现是促肾上腺皮质激素释放因子结合蛋白(CRFBP)的抑制剂肽,可能抑制体重增加。
CRF (6-33) acetate has been found to be a corticotropin-releasing factor binding protein (CRFBP) inhibitor peptide and could probably suppress body weight gain.
定义
酶是用于生化反应的非常有效的催化剂。它们通过提供较低活化能的替代反应途径来加快反应速度。酶作用于底物并产生产物。一些物质降低或什至停止酶的催化活性被称为抑制剂。
发现
1965年,Umezawa H分析了微生物产生的酶抑制剂,并分离出了抑制亮肽素和抗痛药的胰蛋白酶和木瓜蛋白酶,乳糜蛋白酶抑制的胰凝乳蛋白酶,胃蛋白酶抑制素抑制胃蛋白酶,泛磷酰胺抑制唾液酸酶,乌藤酮抑制酪氨酸羟化酶,多巴汀抑制多巴胺3-羟硫基嘧啶和多巴胺3-羟色胺酶酪氨酸羟化酶和多巴胺J3-羟化酶。最近,一种替代方法已应用于预测新的抑制剂:合理的药物设计使用酶活性位点的三维结构来预测哪些分子可能是抑制剂1。已经开发了用于识别酶抑制剂的基于计算机的方法,例如分子力学和分子对接。
结构特征
已经确定了许多抑制剂的晶体结构。已经确定了三种与凝血酶复合的高效且选择性的低分子量刚性肽醛醛抑制剂的晶体结构。这三种抑制剂全部在P3位置具有一个新的内酰胺部分,而对胰蛋白酶选择性最高的两种抑制剂在P1位置具有一个与S1特异性位点结合的胍基哌啶基。凝血酶的抑制动力学从慢到快变化,而对于胰蛋白酶,抑制的动力学在所有情况下都快。根据两步机理2中稳定过渡态络合物的缓慢形成来检验动力学。
埃米尔•菲舍尔(Emil Fischer)在1894年提出,酶和底物都具有特定的互补几何形状,彼此恰好契合。这称为“锁和钥匙”模型3。丹尼尔·科什兰(Daniel Koshland)提出了诱导拟合模型,其中底物和酶是相当灵活的结构,当底物与酶4相互作用时,活性位点通过与底物的相互作用不断重塑。
在众多生物活性肽的成熟过程中,需要由其谷氨酰胺(或谷氨酰胺)前体形成N末端焦谷氨酸(pGlu)。游离形式并与底物和三种咪唑衍生抑制剂结合的人QC的结构揭示了类似于两个锌外肽酶的α/β支架,但有多个插入和缺失,特别是在活性位点区域。几种活性位点突变酶的结构分析为针对QC相关疾病5的抑制剂的合理设计提供了结构基础。
作用方式
酶是催化化学反应的蛋白质。酶与底物相互作用并将其转化为产物。抑制剂的结合可以阻止底物进入酶的活性位点和/或阻止酶催化其反应。抑制剂的种类繁多,包括:非特异性,不可逆,可逆-竞争性和非竞争性。可逆抑制剂 以非共价相互作用(例如疏水相互作用,氢键和离子键)与酶结合。非特异性抑制方法包括最终使酶的蛋白质部分变性并因此不可逆的任何物理或化学变化。特定抑制剂 对单一酶发挥作用。大多数毒药通过特异性抑制酶发挥作用。竞争性抑制剂是任何与底物的化学结构和分子几何结构非常相似的化合物。抑制剂可以在活性位点与酶相互作用,但是没有反应发生。非竞争性抑制剂是与酶相互作用但通常不在活性位点相互作用的物质。非竞争性抑制剂的净作用是改变酶的形状,从而改变活性位点,从而使底物不再能与酶相互作用而产生反应。非竞争性抑制剂通常是可逆的。不可逆抑制剂与酶形成牢固的共价键。这些抑制剂可以在活性位点附近或附近起作用。
功能
工业应用中, 酶在商业上被广泛使用,例如在洗涤剂,食品和酿造工业中。蛋白酶用于“生物”洗衣粉中,以加速蛋白质在诸如血液和鸡蛋等污渍中的分解。商业上使用酶的问题包括:它们是水溶性的,这使得它们难以回收,并且一些产物可以抑制酶的活性(反馈抑制)。
药物分子,许多药物分子都是酶抑制剂,药用酶抑制剂通常以其特异性和效力为特征。高度的特异性和效力表明该药物具有较少的副作用和较低的毒性。酶抑制剂在自然界中发现,并且也作为药理学和生物化学的一部分进行设计和生产6。
天然毒物 通常是酶抑制剂,已进化为保护植物或动物免受天敌的侵害。这些天然毒素包括一些已知最剧毒的化合物。
神经气体( 例如二异丙基氟磷酸酯(DFP))通过与丝氨酸的羟基反应生成酯,从而抑制了乙酰胆碱酯酶的活性位点。
参考
1、Scapin G (2006). Structural biology and drug discovery. Curr. Pharm. Des., 12(17):2087–2097.
2、Krishnan R, Zhang E, Hakansson K, Arni RK, Tulinsky A, Lim-Wilby MS, Levy OE, Semple JE, Brunck TK (1998). Highly selective mechanism-based thrombin inhibitors: structures of thrombin and trypsin inhibited with rigid peptidyl aldehydes. Biochemistry, 37 (35):12094-12103.
3、Fischer E (1894). Einfluss der configuration auf die wirkung der enzyme. Ber. Dt. Chem. Ges., 27:2985–2993.
4、Koshland DE (1958). Application of a theory of enzyme specificity to protein synthesis. PNAS., 44 (2):98–104.
5、Huang KF, Liu YL, Cheng WJ, Ko TP, Wang AH (2005). Crystal structures of human glutaminyl cyclase, an enzyme responsible for protein N-terminal pyroglutamate formation. PNAS., 102(37):13117-13122.
6、Holmes CF, Maynes JT, Perreault KR, Dawson JF, James MN (2002). Molecular enzymology underlying regulation of protein phosphatase-1 by natural toxins. Curr Med Chem., 9(22):1981-1989.
Definition
Enzymes are very efficient catalysts for biochemical reactions. They speed up reactions by providing an alternative reaction pathway of lower activation energy. Enzyme acts on substrate and gives rise to a product. Some substances reduce or even stop the catalytic activities of enzymes are called inhibitors.
Discovery
In 1965, Umezawa H analysed enzyme inhibitors produced by microorganisms and isolated leupeptin and antipain inhibiting trypsin and papain, chymostatin inhibiting chymotrypsin, pepstatin inhibiting pepsin, panosialin inhibiting sialidases, oudenone inhibiting tyrosine hydroxylase, dopastin inhibiting dopamine 3-hydroxylase, aquayamycin and chrothiomycin inhibiting tyrosine hydroxylase and dopamine J3-hydroxylase . Recently, an alternative approach has been applied to predict new inhibitors: rational drug design uses the three-dimensional structure of an enzyme's active site to predict which molecules might be inhibitors 1. Computer-based methods for identifying inhibitor for an enzyme have been developed, such as molecular mechanics and molecular docking.
Structural Characteristics
The crystal structures of many inhibitors have been determined. The crystal structures of three highly potent and selective low-molecular weight rigid peptidyl aldehyde inhibitors complexed with thrombin have been determined. All the three inhibitors have a novel lactam moiety at the P3 position, while the two with greatest trypsin selectivity have a guanidinopiperidyl group at the P1 position that binds in the S1 specificity site. The kinetics of inhibition vary from slow to fast with thrombin and are fast in all cases with trypsin. The kinetics are examined in terms of the slow formation of a stable transition-state complex in a two-step mechanism 2.
Emil Fischer in 1894 suggested that both the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another.This is known as "the lock and key" model 3. Daniel Koshland suggested induced fit model where substrate and enzymes are rather flexible structures, the active site is continually reshaped by interactions with the substrate as the substrate interacts with the enzyme 4.
N-terminal pyroglutamate (pGlu) formation from its glutaminyl (or glutamyl) precursor is required in the maturation of numerous bioactive peptides. The structure of human QC in free form and bound to a substrate and three imidazole-derived inhibitors reveals an alpha/beta scaffold akin to that of two-zinc exopeptidases but with several insertions and deletions, particularly in the active-site region. The structural analyses of several active-site-mutant enzymes provide a structural basis for the rational design of inhibitors against QC-associated disorders 5.
Mode of Action
Enzymes are proteins that catalyze chemical reactions. Enzymes interact with substrate and convert them into products. Inhibitor binding can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. There are a variety of types of inhibitors including: nonspecific, irreversible, reversible - competitive and noncompetitive. Reversible inhibitors bind to enzymes with non-covalent interactions like hydrophobic interactions, hydrogen bonds, and ionic bonds. Non-specific methods of inhibition include any physical or chemical changes which ultimately denature the protein portion of the enzyme and are therefore irreversible. Specific Inhibitors exert their effects upon a single enzyme. Most poisons work by specific inhibition of enzymes. A competitive inhibitor is any compound which closely resembles the chemical structure and molecular geometry of the substrate. The inhibitor may interact with the enzyme at the active site, but no reaction takes place. A noncompetitive inhibitor is a substance that interacts with the enzyme, but usually not at the active site. The net effect of a non competitive inhibitor is to change the shape of the enzyme and thus the active site, so that the substrate can no longer interact with the enzyme to give a reaction. Non competitive inhibitors are usually reversible. Irreversible Inhibitors form strong covalent bonds with an enzyme. These inhibitors may act at, near, or remote from the active site .
Functions
Industrial application, enzymes are widely used commercially, for example in the detergent, food and brewing industries. Protease enzymes are used in 'biological' washing powders to speed up the breakdown of proteins in stains like blood and egg. Problems using enzymes commercially include: they are water soluble which makes them hard to recover and some products can inhibit the enzyme activity (feedback inhibition) .
Drug molecules, many drug molecules are enzyme inhibitors and a medicinal enzyme inhibitor is usually characterized by its specificity and its potency. A high specificity and potency suggests that a drug will have fewer side effects and less toxic. Enzyme inhibitors are found in nature and are also designed and produced as part of pharmacology and biochemistry 6.
Natural poisons are often enzyme inhibitors that have evolved to defend a plant or animal against predators. These natural toxins include some of the most poisonous compounds known.
Nerve gases such as diisopropylfluorophosphate (DFP) inhibit the active site of acetylcholine esterase by reacting with the hydroxyl group of serine to make an ester.
References
Scapin G (2006). Structural biology and drug discovery. Curr. Pharm. Des., 12(17):2087–2097.
Krishnan R, Zhang E, Hakansson K, Arni RK, Tulinsky A, Lim-Wilby MS, Levy OE, Semple JE, Brunck TK (1998). Highly selective mechanism-based thrombin inhibitors: structures of thrombin and trypsin inhibited with rigid peptidyl aldehydes. Biochemistry, 37 (35):12094-12103.
Fischer E (1894). Einfluss der configuration auf die wirkung der enzyme. Ber. Dt. Chem. Ges., 27:2985–2993.
Koshland DE (1958). Application of a theory of enzyme specificity to protein synthesis. PNAS., 44 (2):98–104.
Huang KF, Liu YL, Cheng WJ, Ko TP, Wang AH (2005). Crystal structures of human glutaminyl cyclase, an enzyme responsible for protein N-terminal pyroglutamate formation. PNAS., 102(37):13117-13122.
Holmes CF, Maynes JT, Perreault KR, Dawson JF, James MN (2002). Molecular enzymology underlying regulation of protein phosphatase-1 by natural toxins. Curr Med Chem., 9(22):1981-1989.

ACTH的定义
促肾上腺皮质激素(ACTH、Adrenocorticotropic hormone)或促肾上腺皮质激素是垂体前叶产生的一种激素,它刺激肾上腺皮质。
促肾上腺皮质激素(ACTH),也称为促肾上腺皮质激素,是一种由垂体前叶腺产生和分泌的多肽促性激素。它是下丘脑-垂体-肾上腺轴的重要组成部分,通常是在对生物压力的反应中产生的(连同下丘脑的促肾上腺皮质激素释放激素)。它的主要作用是增加皮质类固醇的产生和释放,以及顾名思义,是肾上腺皮质的皮质醇。
ACTH是由前opiomelanocortin(pre-POMC)合成的。翻译过程中信号肽的去除产生了241个氨基酸的多肽POMC,在被内肽酶进行蛋白水解切割之前,它会经历一系列的翻译后修饰,例如磷酸化和糖基化,以产生具有不同生理活性的各种多肽片段。这些片段包括NPP,促黑素Gamma(γ-MSH),潜在肽,促肾上腺皮质激素(促肾上腺皮质激素或ACTH),促黑素Alpha(促黑素细胞激素或α-MSH),促肾上腺皮质激素样中间肽(CLIP),促肾上腺皮质激素Beta (β-LPH),脂蛋白Gamma(γ-LPH),促黑素β(β-MSH),β-内啡肽和Met-脑啡肽。POMC,
为了调节ACTH的分泌,该轴内分泌的许多物质表现出缓慢/中间和快速的反馈环活性。肾上腺皮质分泌的糖皮质激素起抑制下丘脑CRH分泌的作用,进而降低了ACTH的垂体前叶分泌。糖皮质激素也可能抑制POMC基因转录和肽合成的速率。后者是一个缓慢的反馈循环的例子,它的工作时间从几小时到几天不等,而前者的工作时间则在几分钟到几分钟。
ACTH还与许多生物体的昼夜节律有关。ACTH在人血中的半衰期约为十分钟。
相关肽
六个相关肽包括较小的生物活性片段(激素),这些片段是通过对前opiomelanocortin多蛋白(POMC)进行差分加工而从共同的前体衍生而来的。ACTH,促肾上腺皮质激素样中间叶蛋白[CLIP],β-内啡肽,γ-脂蛋白[yLPH],脑啡肽和α-黑素蛋白[aMSH] 1。
ACTH的发现
1930年代首次研究了ACTH的性质。1933年,由詹姆斯·科利普(James Collip),赫伯特·埃文斯(Herbert Evans)和贝玛尔多·侯赛(Bemardo Houssay)领导的研究小组使用垂体提取物刺激肾上腺皮质。美国生物化学家Choh Hao Li是1943年分离ACTH并在1963年对其进行合成的几位科学家之一。
ACTH的结构特征
ACTH的分子量为4541.3 K Da 2。它是由39个氨基组成的直链肽分子。前24个氨基酸和后7个氨基酸相同,而第25至32个氨基酸略有不同。只有前20个氨基酸才能发挥全部活性,称为活性中心。
ACTH的作用机制
ACTH进入全身循环并与位于肾上腺皮质细胞和皮肤表面的特定高亲和力受体结合。ACTH受体是七种跨膜的G蛋白偶联受体3,在配体结合后会经历构象变化,从而刺激腺苷酸环化酶,从而导致细胞内cAMP的增加和蛋白激酶A的激活。最终导致类固醇生成的刺激。
ACTH的功能
ACTH的主要功能是刺激肾上腺皮质分泌一组类固醇激素,称为糖皮质激素,盐皮质激素和雄激素类固醇。糖皮质激素可控制人体对糖的使用,并在压力时刻帮助调节生物功能。它刺激胆固醇转化为孕烯醇酮,这是所有类固醇激素的前体。它用于治疗类风湿关节炎,溃疡性结肠炎,肝炎和减轻疼痛。它们通过促进必需的蛋白质在特定于记忆的位点4合成所需的蛋白质,在记忆处理中起主要作用。
ACTH的的文献参考
1、Funkelstein L, Toneff T, Mosier C, Hwang SR, Beuschlein F, Lichtenauer UD, Reinheckel T, Peters C, Hook V (2008). Major role of cathepsin L for producing the peptide hormones ACTH, beta-endorphin, and alpha-MSH, illustrated by protease gene knockout and expression. J Biol Chem., 283(51):35652-35659.
2、Lee TH, Lerner AB, Buettner-Janusch V (1961). On the structure of human corticotropin (adrenocorticotropic hormone). J. Biol. Chem., 236:2970-2974.
3、Mountjoy KG, Robbins LS, Mortrud MT, Cone RD (1992). The cloning of a family of genes that encode the melanocortin receptors. Science, 257:248–1251.
4、Flood JF, Jarvik ME, Bennett EL, Orme AE (1976). Effects of ACTH peptide fragments on memory formation. Pharmacol Biochem Behav., 5:41-51.
S C Heinrichs, et al. Dissociation of arousal-like from anxiogenic-like actions of brain corticotropin-releasing factor receptor ligands in rats. Behav Brain Res. 2001 Jul;122(1):43-50. : https://pubmed.ncbi.nlm.nih.gov/11287075
S C Heinrichs, et al. Selective stimulatory actions of corticotropin-releasing factor ligands on correlates of energy balance. Physiol Behav. 2001 Sep 1-15;74(1-2):5-13. : https://pubmed.ncbi.nlm.nih.gov/11564446/





