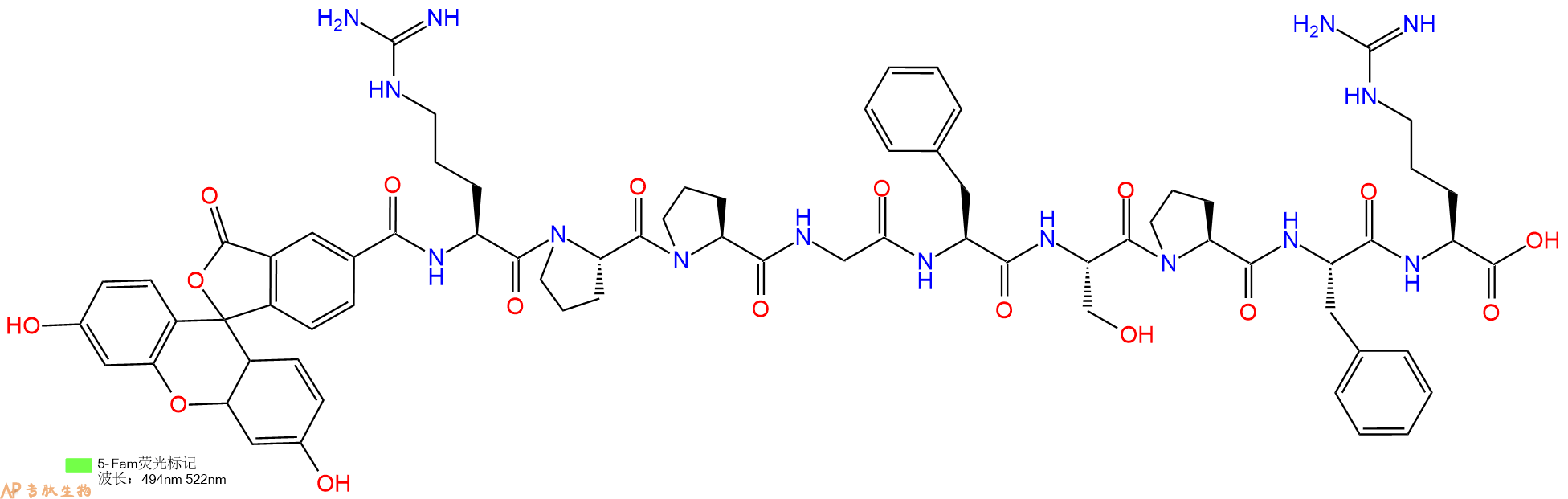
| 编号: | 186819 |
| 中文名称: | 缓激肽Fam-Bradykinin |
| 英文名: | Fam-Bradykinin |
| 单字母: | 5FAM-RPPGFSPFR-OH |
| 三字母: | 5FAM N端标记5-FAM -Arg精氨酸 -Pro脯氨酸 -Pro脯氨酸 -Gly甘氨酸 -Phe苯丙氨酸 -Ser丝氨酸 -Pro脯氨酸 -Phe苯丙氨酸 -Arg精氨酸 -OHC端羧基 |
| 氨基酸个数: | 9 |
| 分子式: | C71H83O17N15 |
| 平均分子量: | 1418.51 |
| 精确分子量: | 1417.61 |
| 等电点(PI): | - |
| pH=7.0时的净电荷数: | 2 |
| 平均亲水性: | 0.26 |
| 疏水性值: | -1.04 |
| 外观与性状: | 淡黄色粉末状固体 |
| 消光系数: | - |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 纯度: | 95%、98% |
| 盐体系: | 可选TFA、HAc、HCl或其它 |
| 生成周期: | 2-3周 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | FAM、FITC修饰肽 缓激肽(Bradykinin) |
多肽荧光标记由于没有放射性,实验操作简单。因此,目前在生物学研究中多肽荧光标记应用非常广泛,多肽荧光标记方法与荧光试剂的结构有关系,对于有游离羧基的采用的方法与接多肽反应相同,也采用HBTU/HOBt/DIEA方法连接。 在N端标记FITC的多肽需经历环化作用来形成荧光素,通常会伴有最后一个氨基酸的去除,但当有一个间隔器如氨基己酸,或者是通过非酸性环境将目的多肽从树脂上切下来时,这种情况可避免在切割的过程中被TFA切割掉。
人们利用利用荧光标记的多肽来检测目标蛋白的活性,并将 其发展的高通量活性筛选方法应用于疾病治疗靶点蛋白的药物筛选和药物开发(例如,各种激 酶、磷酸酶、肽酶等)。
专肽生物能够提供技术成熟的各种荧光标记多肽。
下面是一些常见的多肽修饰荧光物质结构:
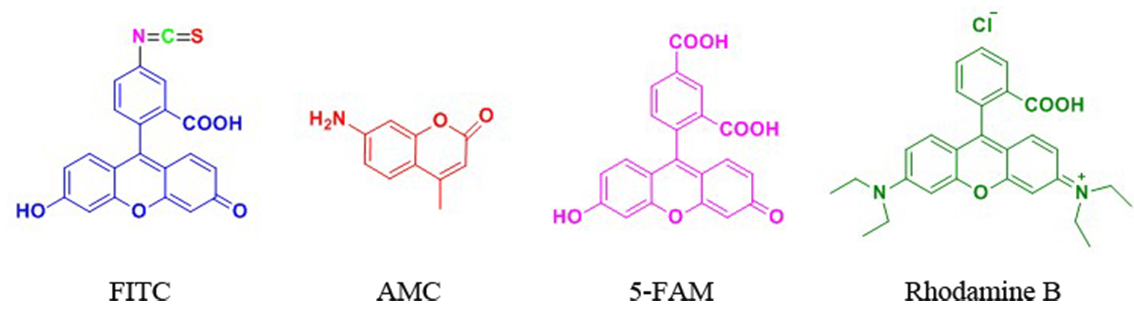
FITC标记
FITC(异硫氰酸荧光素)具有比较高的活性,我们公司可以通过两种方式将FITC标记于多肽 上:(1) 将FITC标记于赖氨酸(Lys)或被选择性地脱保护的鸟氨酸(ornithine)侧链氨基 上;(2) 将FITC标记于多肽N端氨基。
当在N端标记时,建议在最后一个氨基和由异硫氰酸酯与氨基反应产生的硫脲键之间引入 烷基间隔器(alkyl spacer),如氨基己酸(Ahx)。链接切割需要酸性环境,在N端标记FITC 的多肽需经历环化作用来形成荧光素,通常会伴有最后一个氨基酸的去除,但当有一个间隔器 如氨基己酸,或者是通过非酸性环境将目的肽从树脂上切下来时,这种情况可避免。空间位阻 被认为是在荧光染料前使用Ahx的主要原因,而不是为什么FITC不能直接偶联在多肽上的原因。
Ahx或b-Ala均可作为间隔器用于FITC标记的多肽上。

| 荧光修饰中文名称 | N端 | N端带有linker |
| 生物素标记多肽 | Biotin- | Biotin-Ahx- |
| 异硫氰酸荧光素 | FITC- | FITC-Ahx- |
| 5-羧基荧光素 | 5-FAM- | 5-FAM-Ahx- |
| 丹磺酰荧光素 | Dansyl- | Dansyl-Ahx- |
| 5-羧基四甲基罗丹明 | TMR- (TAMRA-) | TMR-Ahx- (TAMRA-Ahx-) |
| 多肽N端 | 多肽序列中间 | N端带有linker |
| 生物素标记多肽 | Biotin- | 多肽C端 |
| Lys(Biotin)- | -Lys(Biotin)-- | -Lys(Biotin) |
| Lys(FITC)- | -Lys(FITC)- | -Lys(FITC) |
| Lys(5-FAM)- | -Lys(5-FAM)- | -Lys(5-FAM) |
| Lys(Dansyl)- | -Lys(Dansyl)- | -Lys(Dansyl) |
| Lys(TMR)- | -Lys(TMR)- | -Lys(TMR) |
| Lys(Dnp)- | -Lys(Dnp)- | -Lys(Dnp) |
专肽常做的荧光物质的激发光波长和发射光波长。可供参考选择:
| 荧光基团 | Ex(nm) | Em(nm) | 荧光基团 | Ex(nm) | Em(nm) |
| 羟基香豆素 | 325 | 386 | R-phycoerythrin (PE) (489) | 565 | 578 |
| 丹磺酰氯 | 340 | 578 | Rhodamine Red-X | 560 | 580 |
| AMC | 345 | 445 | Tamara | 565 | 580 |
| 甲氧基香豆素 | 360 | 410 | Alexa fluor 555 | 556 | 573 |
| Alexa fluor 系列 | 345 | 442 | Alexa fluor 546 | 556 | 573 |
| 氨基香豆素 | 350 | 445 | Rox | 575 | 602 |
| Dabcyl | 453 | - | Alexa fluor 568 | 578 | 603 |
| Cy2 | 490 | 510 | Texas Red | 589 | 615 |
| FAM | 495 | 517 | Alexa fluor 594 | 590 | 617 |
| Alexa fluor 488 | 494 | 517 | Alexa fluor | 621 | 639 |
| FITC | 495 | 519 | Alexa fluor 633 | 650 | 668 |
| Alexa fluor 430 | 430 | 545 | Cy5 (625) | 650 | 670 |
| 5-FAM | 492 | 518 | Alexa fluor 660 | 663 | 690 |
| Alexa fluor 532 | 530 | 530 | Cy5.5 | 675 | 694 |
| HEX | 535 | 556 | TruRed | 490; 675 | 695 |
| 5-TAMRA | 542 | 568 | Alexa fluor 680 | 679 | 702 |
| Cy3 | 550 | 570 | Cy7 | 743 | 767 |
| TRITC | 547 | 572 | Cy3.5 | 581 | 596 |
Definition
Bradykinin is a nonapeptide that is mainly found in animal preparations that are treated with the venom of the snake, Bothrops jararaca1,2. It dialates blood vessels that in turn leads to decrease in blood pressure2. Bradykinin analogs are slightly modified structural derivatives of bradykinin that perform similar functions as bradykinin3.
Discovery
Bradykinin was discovered in the blood plasma of animals that were treated with the venom from the Brazilian snake, Bothrops jararaca1,2. The discovery was part of a study that was related to toxicology of snake bites. Bradykinin analogs were synthesized by solid-phase techniques in 1975 and their function was studied in rats and rabbits3.
Classification
Bradykinin is a 9 amino acid peptide that belongs to the kinin family of proteins4. It has homologs in several animals including other snakes, frog, dog and humans4.
Structural Characteristics
Bradykinin has the sequence Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg3. Several analogs of bradykinin have been synthesized. They are also nanopeptides containing substitutions of various amino acids of bradykinin. For example two analogs of bradykinin were synthesized one with 7-beta-homo-L-proline and the other with 8-beta-homo-L-phenylalanine substitutions3. It was found that both of them are resistant to enzymatic degradation3.
Mode of action
Bradykinin binds to two different kinin G protein coupled receptors- B1 and B25. Upon binding to these receptors it induces conversion of GTP to GDP which in turn triggers the conversion ATP to cAM which then acts as a second messenger resulting in the activation of genes. B1 receptor is expressed as a result of tissue injury and is found to play a role in inflammation while B2 receptor participates in the vasodilatory role of bradykinin5,6. Bradykinin analogs function is a similar fashion although depending on their structure they might have varying affinities to the receptors compared to bradykinin7. Also analogs of bradykinin have been synthesized that are specific to one of these receptors7.
Functions
Bradykinin is a potent endothelium-dependent vasodilator, causes contraction of non-vascular smooth muscle, increases vascular permeability and also is involved in the mechanism of pain. Bradykinin also causes natriuresis, contributing to a drop in blood pressure8. Bradykinin raises internal calcium levels in neocortical astrocytes causing them to release glutamate9. Overactivation of bradykinin is thought to play a role in a rare disease called Hereditary Angioedema, also known as Hereditary Angio-Neurotic Edema10.
Some analogs of bradykinin have been found to have prolonged hypotensive action compared to bradykinin (Eg: beta-H-Pro-bradykinin)3. Some analogs have relative or even lower potencies compared to bradykinin (Eg: HArg1-Bradykinin and HArg9 Bradykinin)7. Other analogs have been studied for their potential of finding bradykinin antagonists that might be useful in the treatment of angio-neurotic edema.
References
1. Partridge, SM (1948). (Title or abstract not available), Biochem. J., 42, 238.
2. Allen PK, Kusumam J, Yoji S, Yoshitaka N, Berhane G, Sesha R and Michael S (1998). Bradykinin formation: Plasma and tissue pathways and cellular interactions. Clinical reviews in allergy and immunology, 16, 4, 403-429.
3. Ondetti MA, Engel SL (1975). Bradykinin analogs containing beta.-homoamino acid, J. Med. Chem.,18 (7), 761–763.
4. Roseli A, Gomes S, Jair RC, Luis J and Valdemar H (1996). Met-Lys-Bradykinin-Ser, the kinin released from human kininogen by human pepsin. Immunopharmacology, 32, 76-79.
5. Peter GM, Amrita A, and Mauro P (2000). Association between Kinin B1 Receptor Expression and Leukocyte Trafficking across Mouse Mesenteric Postcapillary Venules. J Exp. Med., 192, 367-380.
6. Duchene J, Lecomte F, Ahmed S, Cayla C, Pesquero J, Bader M, Perretti M and Ahluwalia A, (2007). A Novel Inflammatory Pathway Involved in Leukocyte Recruitment: Role for the Kinin B1 Receptor and the Chemokine CXCL5. J Immunol., 179, 4849-4856.
7. Max ES, Phyllis AL (1974). Synthesis and pharmacology of homoarginine bradykinin analog. J. Med. Chem., 17 (11), pp 1227–1228.
8. Dendorfer A, Wolfrum S, Wagemann M, Qadri F, Dominiak P, (2001). Pathways of bradykinin degradation in blood and plasma of normotensive and hypertensive rats. Am J Physiol Heart Circ Physiol., 280:H2182
9. Kuoppala A, Lindstedt KA, Saarinen J, Kovanen PT, Kokkonen JO (2000). Inactivation of bradykinin by angiotensin-converting enzyme and by carboxypeptidase N in human plasma. Am J Physiol Heart Circ Physiol, 278(4):H1069-74.
10. Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G (2007). Nonallergic angioedema: role of bradykinin. Allergy, 62(8):842-56.





