| 编号: | 191612 |
| 中文名称: | a-Neo-Endorphin Analog |
| 英文名: | a-Neo-Endorphin Analog |
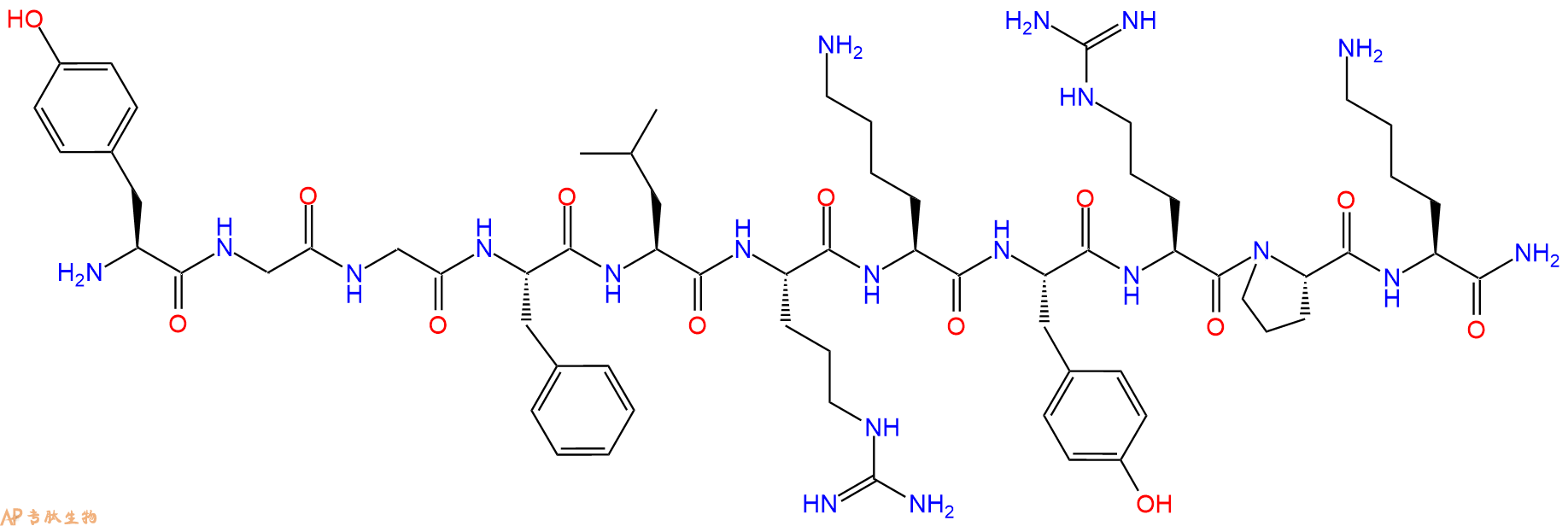
| 单字母: | H2N-YGGFLRKYRPK-NH2 |
| 三字母: | H2N N端氨基 -Tyr酪氨酸 -Gly甘氨酸 -Gly甘氨酸 -Phe苯丙氨酸 -Leu亮氨酸 -Arg精氨酸 -Lys赖氨酸 -Tyr酪氨酸 -Arg精氨酸 -Pro脯氨酸 -Lys赖氨酸 -NH2C端酰胺化 |
| 氨基酸个数: | 11 |
| 分子式: | C66H102N20O13 |
| 平均分子量: | 1383.64 |
| 精确分子量: | 1382.79 |
| 等电点(PI): | - |
| pH=7.0时的净电荷数: | 6.97 |
| 平均亲水性: | 0.1125 |
| 疏水性值: | -1.31 |
| 外观与性状: | 白色粉末状固体 |
| 消光系数: | 2980 |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 纯度: | 95%、98% |
| 盐体系: | 可选TFA、HAc、HCl或其它 |
| 生成周期: | 2-3周 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 内啡肽(Endorphin) |
Definition
Endorphins are small neuropeptides that are produced by the body and act to reduce pain hence, the name endorphin (a shortened version of endogenous morphine). The term "enkephalin" (meaning literally "in the head") is also applied to endorphins, but usually refers to smaller molecules that have pain-relieving properties 1.
Related Peptides
There are 3 types of Endorphins:
Enkephalins: Met- and Leu-
Endorphins
Dynorphins
Endorphins are neuropeptides that can range from 2 to 39 amino acids in length. Neuropeptides are peptide molecules produced and released in the nervous system that act like transmitters 2. There are three different neuropeptide sequences including enkephalins, endorphins, and dynorphins 3
Discovery
In 1975, John Hughes and Hans W. Kosterlitz of the University of Aberdeen isolated two naturally occurring peptides in the brain that bound tightly to the opiate receptors and named them enkephalins. The endorphin molecule was subsequently isolated from the pituitary gland 4.
Structural Characteristics
Four distinct groups of endorphins have been identified to date. They have been termed: a-endorphin, a polypeptide with 16 residues; ß-endorphin, a polypeptide with 31 residues; ?-endorphin, a polypeptide with 17 residues; and S-endorphin, a polypeptide with 27 residues. These different types of endorphins, like all known polypeptide hormones, are synthesized in a "prepro" form that is one gigantic polypeptide with a signal sequence and additional sequences that are cleaved out during posttranslational maturation of the polypeptide. The most interesting example of this is the pituitary multihormone precursor termed pro-opiomelanocortin that contains the sequences for ß-lipotropin, melanocyte-stimulating hormone (MSH), endorphins, enkephalins, and adrenocorticotropic hormone (ACTH). After synthesis, this peptide is cleaved in the pituitary to generate ACTH and ß-lipotropin, while processing in the central nervous system produces endorphins and enkephalins, along with some other products 5.
Mode of Action
Receptors enable endorphins to perform their specific function. Opioid receptors are large protein molecules embedded in the semi-fluid matrix of the cell membrane of the receiving neuron. The surface of the receptor protein contains a region that is the precise size and shape to match the structure of the endorphin molecule. The endorphin molecule precisely fits into the specific receptor site. The binding of the neuropeptide with its specific receptor (opioid receptor) alters the three-dimensional shape of the receptor protein, thereby causing a neuron to be excited or inhibited6. As in the case of endorphins, inhibition of the neuron will reduce the release of substance P. In other words, the opioid receptor translates the precise messages encoded by the molecular structure of the endorphin molecule into a specific physiological response. Thus, receptors act as a control mechanism thereby regulating the function of endorphins 7.
Functions
Endorphins are not considered to be neurotransmitter molecules, but are instead classified as neuromodulatory, that is, they modify the action of neurotransmitters through a number of effects associated with pain or pleasure. Endorphins exhibit a number of neurological effects associated with the relief of pain. The administration of exogenous endorphins (those prepared outside the body) stimulates the release of many other hormones including prolactin, ACTH, and antidiuretic hormone. The analgesic effects of morphine are commonly believed to be caused by binding to receptor sites for endorphins, but few beneficial effects of treatment with exogenous endorphins have been reported. Early speculations concerning the function of endorphins suggested that they were natural painkillers that the body produced to alleviate pain in circumstances requiring an individual to continue functioning in spite of injury or stress. Examples of such situations might include childbirth, exercise, and combat. Several procedures that treat chronic pain (acupuncture, direct electrical stimulation of the brain and even hypnosis) may act by inducing the release of enkephalins or endorphins in the brain and spinal cord. This hypothesis is based on the finding that the effectiveness of treating pain implemented by these procedures is blocked by administration of naloxone, a drug that specifically blocks the binding of morphine to the opiate receptor 1.
References
Book: Textbook of Biochemistry: With Clinical Correlations by Devlin TM.
Book: Animal Physiology by Eckert R.
Book: Neurobiology by Shepherd GM.
Book: The Brain by Iverson L.
Book: Molecular Expressions: Exploring the World of Optics and Microscopy Michael WD.
Book: Neural and Integrative Animal Physiology by Prosser CL.
Book:. Neuroscience by Barker RA
多肽H2N-Tyr-Gly-Gly-Phe-Leu-Arg-Lys-Tyr-Arg-Pro-Lys-NH2的合成步骤:
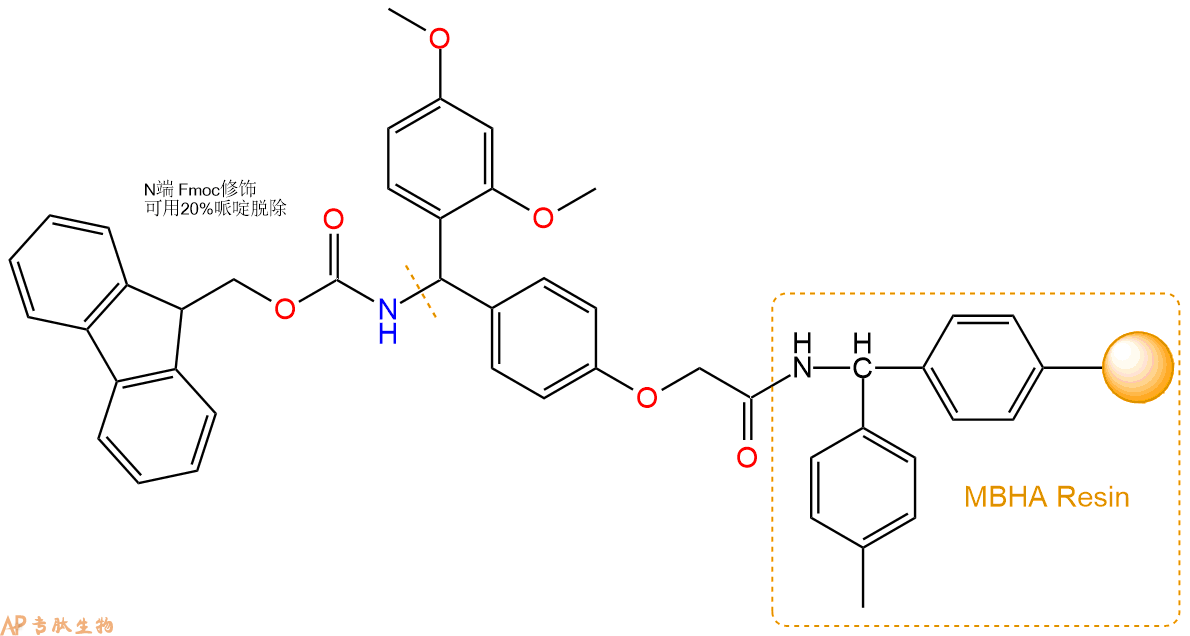
1、合成MBHA树脂:取若干克的MBHA树脂(如初始取代度为0.5mmol/g)和1倍树脂摩尔量的Fmoc-Linker-OH加入到反应器中,加入DMF,搅拌使氨基酸完全溶解。再加入树脂2倍量的DIEPA,搅拌混合均匀。再加入树脂0.95倍量的HBTU,搅拌混合均匀。反应3-4小时后,用DMF洗涤3次。用2倍树脂体积的10%乙酸酐/DMF 进行封端30分钟。然后再用DMF洗涤3次,甲醇洗涤2次,DCM洗涤2次,再用甲醇洗涤2次。真空干燥12小时以上,得到干燥的树脂{Fmoc-Linker-MHBA Resin},测定取代度。这里测得取代度为 0.3mmol/g。结构如下图:
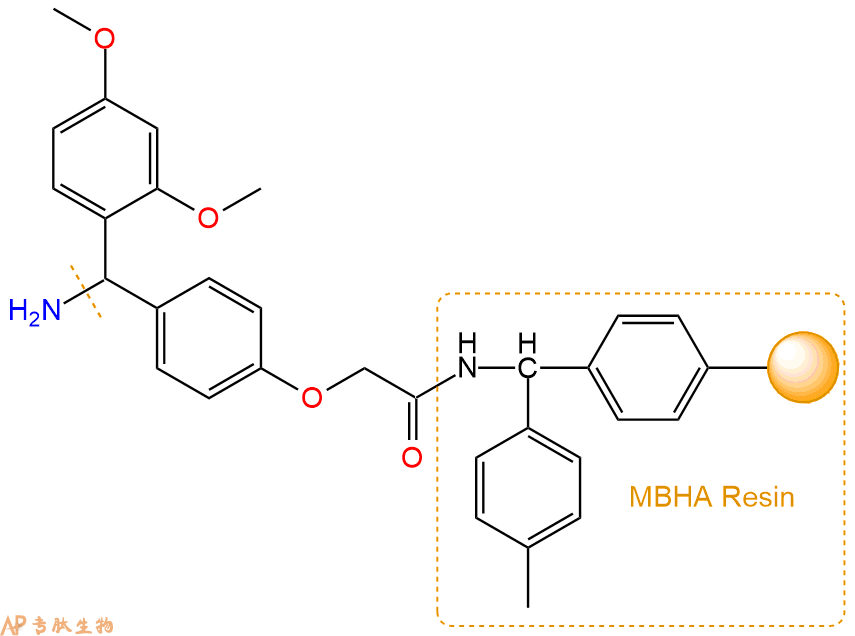
2、脱Fmoc:取2.91g的上述树脂,用DCM或DMF溶胀20分钟。用DMF洗涤2遍。加3倍树脂体积的20%Pip/DMF溶液,鼓氮气30分钟,然后2倍树脂体积的DMF 洗涤5次。得到 H2N-Linker-MBHA Resin 。(此步骤脱除Fmoc基团,茚三酮检测为蓝色,Pip为哌啶)。结构图如下:
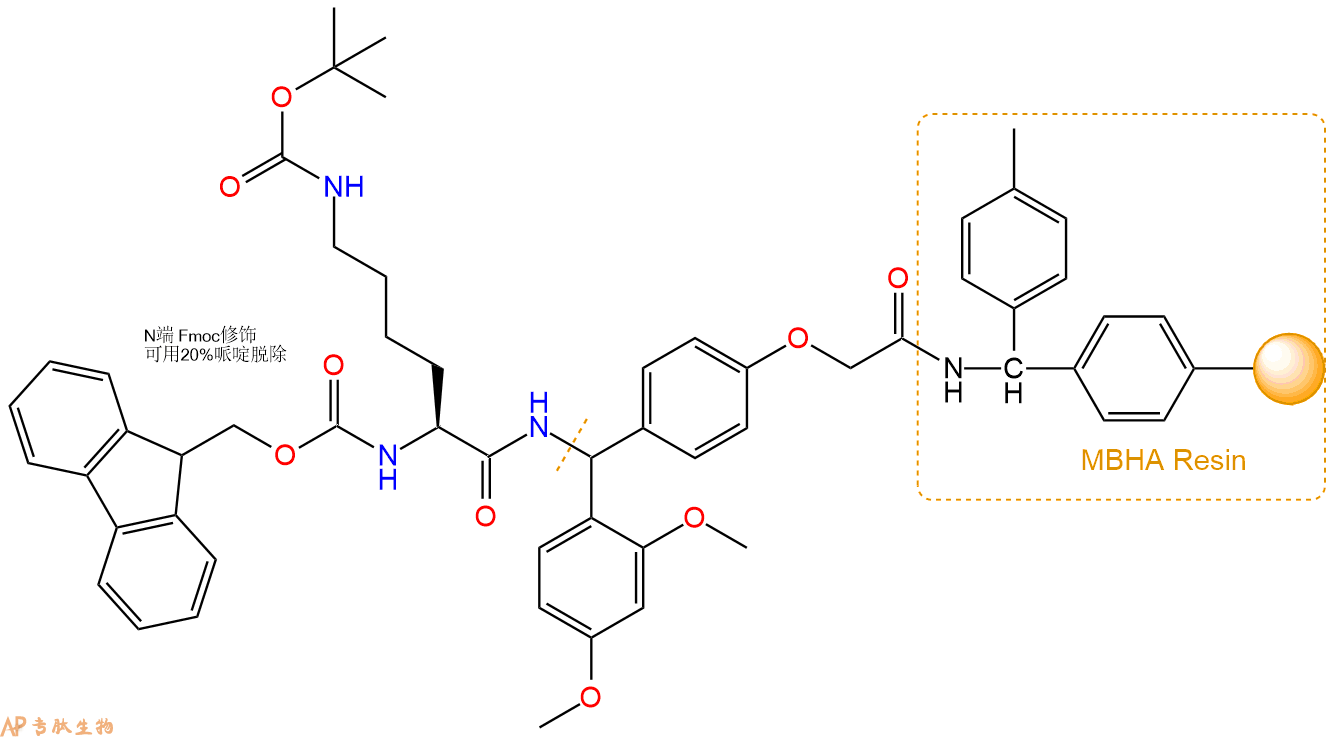
3、缩合:取2.62mmol Fmoc-Lys(Boc)-OH 氨基酸,加入到上述树脂里,加适当DMF溶解氨基酸,再依次加入5.24mmol DIPEA,2.49mmol HBTU。反应30分钟后,取小样洗涤,茚三酮检测为无色。用2倍树脂体积的DMF 洗涤3次树脂。(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。得到Fmoc-Lys(Boc)-Linker-MBHA Resin。氨基酸:DIPEA:HBTU:树脂=3:6:2.85:1(摩尔比)。结构图如下:
4、依次循环步骤二、步骤三,依次得到
H2N-Lys(Boc)-Linker-MBHA Resin
Fmoc-Pro-Lys(Boc)-Linker-MBHA Resin
H2N-Pro-Lys(Boc)-Linker-MBHA Resin
Fmoc-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
H2N-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
Fmoc-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
H2N-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
Fmoc-Lys(Boc)-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
H2N-Lys(Boc)-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
Fmoc-Arg(Pbf)-Lys(Boc)-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
H2N-Arg(Pbf)-Lys(Boc)-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
Fmoc-Leu-Arg(Pbf)-Lys(Boc)-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
H2N-Leu-Arg(Pbf)-Lys(Boc)-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
Fmoc-Phe-Leu-Arg(Pbf)-Lys(Boc)-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
H2N-Phe-Leu-Arg(Pbf)-Lys(Boc)-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
Fmoc-Gly-Phe-Leu-Arg(Pbf)-Lys(Boc)-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
H2N-Gly-Phe-Leu-Arg(Pbf)-Lys(Boc)-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
Fmoc-Gly-Gly-Phe-Leu-Arg(Pbf)-Lys(Boc)-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
H2N-Gly-Gly-Phe-Leu-Arg(Pbf)-Lys(Boc)-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
Fmoc-Tyr(tBu)-Gly-Gly-Phe-Leu-Arg(Pbf)-Lys(Boc)-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin
以上中间结构,均可在专肽生物多肽计算器-多肽结构计算器中,一键画出。
最后再经过步骤二得到 H2N-Tyr(tBu)-Gly-Gly-Phe-Leu-Arg(Pbf)-Lys(Boc)-Tyr(tBu)-Arg(Pbf)-Pro-Lys(Boc)-Linker-MBHA Resin,结构如下:
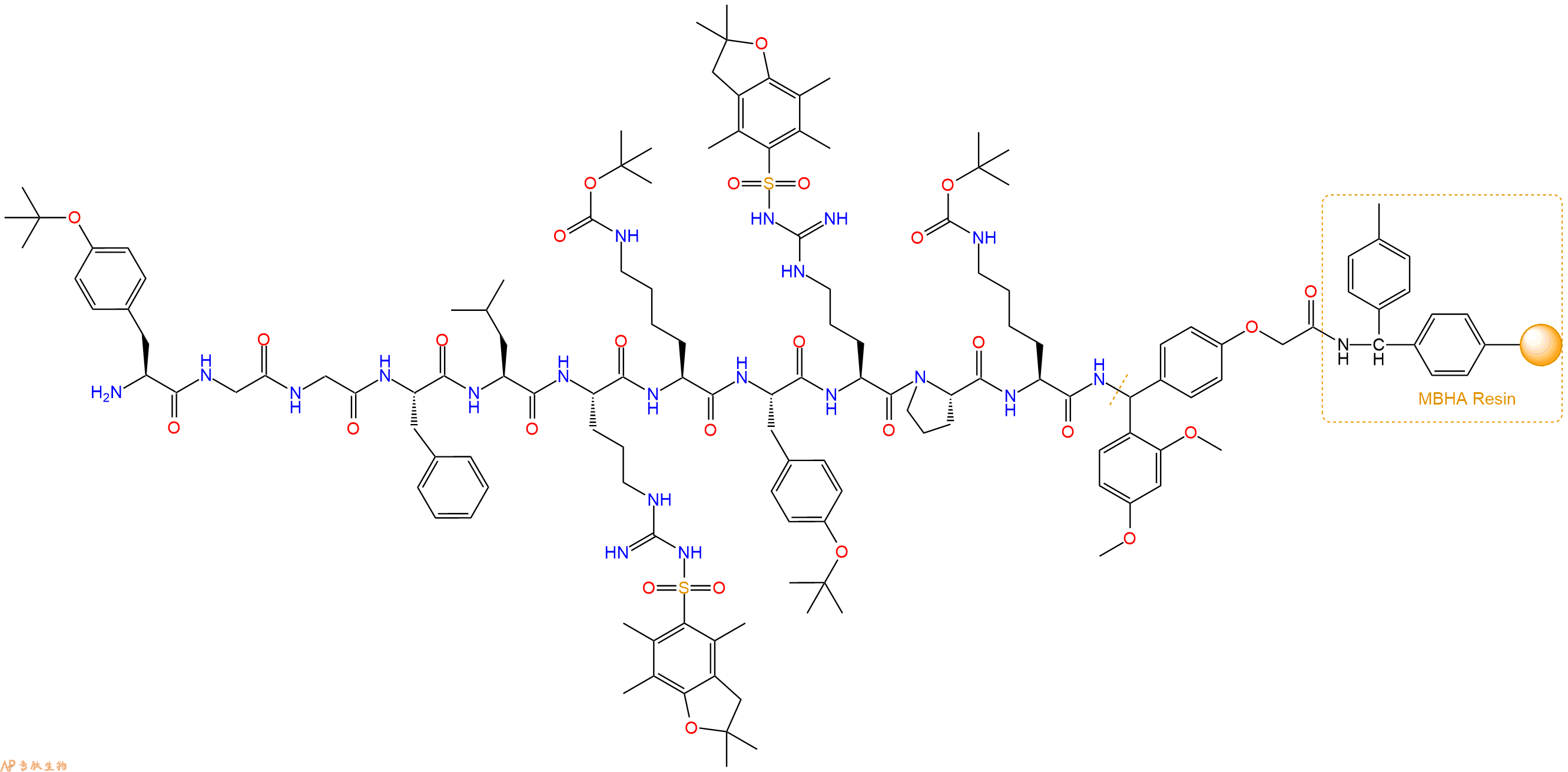
5、切割:6倍树脂体积的切割液(或每1g树脂加8ml左右的切割液),摇床摇晃 2小时,过滤掉树脂,用冰无水乙醚沉淀滤液,并用冰无水乙醚洗涤沉淀物3次,最后将沉淀物放真空干燥釜中,常温干燥24小试,得到粗品H2N-Tyr-Gly-Gly-Phe-Leu-Arg-Lys-Tyr-Arg-Pro-Lys-NH2。结构图见产品结构图。
切割液选择:1)TFA:H2O=95%:5%
2)TFA:H2O:TIS=95%:2.5%:2.5%
3)三氟乙酸:茴香硫醚:1,2-乙二硫醇:苯酚:水=87.5%:5%:2.5%:2.5%:2.5%
(前两种适合没有容易氧化的氨基酸,例如Trp、Cys、Met。第三种适合几乎所有的序列。)
6、纯化冻干:使用液相色谱纯化,收集目标峰液体,进行冻干,获得蓬松的粉末状固体多肽。不过这时要取小样复测下纯度 是否目标纯度。
7、最后总结:
杭州专肽生物技术有限公司(ALLPEPTIDE https://www.allpeptide.com)主营定制多肽合成业务,提供各类长肽,短肽,环肽,提供各类修饰肽,如:荧光标记修饰(CY3、CY5、CY5.5、CY7、FAM、FITC、Rhodamine B、TAMRA等),功能基团修饰肽(叠氮、炔基、DBCO、DOTA、NOTA等),同位素标记肽(N15、C13),订书肽(Stapled Peptide),脂肪酸修饰肽(Pal、Myr、Ste),磷酸化修饰肽(P-Ser、P-Thr、P-Tyr),环肽(酰胺键环肽、一对或者多对二硫键环),生物素标记肽,PEG修饰肽,甲基化修饰肽等。
以上所有内容,为专肽生物原创内容,请勿发布到其他网站上。





