类胰岛素生长因子I 30 至 41 的氨基酸片段。IGF-I 对生长激素的活性有着重要的作用,但是IGF-I本身也具有一些自身的特性,如合成代谢、抗氧化、抗炎和细胞保护作用。
编号:154171
CAS号:82177-09-1
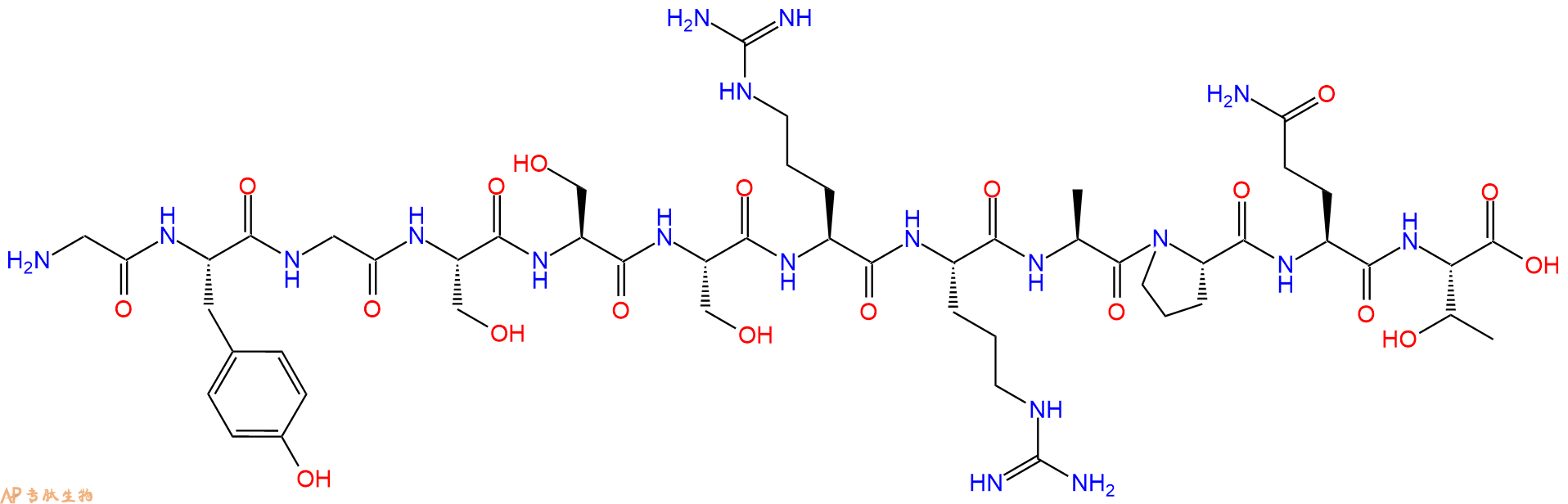
单字母:H2N-GYGSSSRRAPQT-OH
| 编号: | 154171 |
| 中文名称: | Insulin-Like Growth Factor I (30-41)、IGF-I 30-41 |
| 英文名: | Insulin-Like Growth FactorI (30-41) |
| 英文同义词: | IGF-I 30-41 |
| CAS号: | 82177-09-1 |
| 单字母: | H2N-GYGSSSRRAPQT-OH |
| 三字母: | H2N N端氨基 -Gly甘氨酸 -Tyr酪氨酸 -Gly甘氨酸 -Ser丝氨酸 -Ser丝氨酸 -Ser丝氨酸 -Arg精氨酸 -Arg精氨酸 -Ala丙氨酸 -Pro脯氨酸 -Gln谷氨酰胺 -Thr苏氨酸 -OHC端羧基 |
| 氨基酸个数: | 12 |
| 分子式: | C51H83N19O19 |
| 平均分子量: | 1266.32 |
| 精确分子量: | 1265.61 |
| 等电点(PI): | - |
| pH=7.0时的净电荷数: | 3.97 |
| 平均亲水性: | 0.31111111111111 |
| 疏水性值: | -1.43 |
| 外观与性状: | 白色粉末状固体 |
| 消光系数: | 1490 |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 纯度: | 95%、98% |
| 盐体系: | 可选TFA、HAc、HCl或其它 |
| 生成周期: | 2-3周 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 生长激素及生长因子 胰岛素样生长因子(Insulin-Like Growth Factors, IGF) |
IGF-I (30-41) 是类胰岛素生长因子I 30 至 41 的氨基酸片段。IGF-I 对生长激素的活性有着重要的作用,但是IGF-I本身也具有一些自身的特性,如合成代谢、抗氧化、抗炎和细胞保护作用。
IGF-I (30-41) is amino acids 30 to 41 fragment of Insulin-like Growth Factor I (IGF-I). IGF-I is partly responsible for systemic GH activities although it possesses a wide number of own properties (anabolic, antioxidant, anti-inflammatory and cytoprotective actions).
IGF-I 30-41醋酸盐是胰岛素样生长因子I(IGF-I)的30-41个氨基酸片段。IGF-I具有合成代谢、抗氧化、抗炎和细胞保护特性。
IGF-I 30-41 acetate is a 30-41 amino acid fragment of insulin-like growth factor I(IGF-I). IGF-I has anabolic, antioxidant, anti-inflammatory and cell-protective properties.
定义
生长激素(GH)或生长激素在垂体的前部产生。GH的许多作用是由肝脏和其他组织分泌的胰岛素样生长因子1(IGF-1)介导的。
发现
生长激素释放激素(GHRH)基因产生的前体分子包含GHRH和被称为GHRH相关肽(GHRH-RP)的羧基末端肽。像GHRH一样,这种肽在体外和体内刺激干细胞因子(SCF)的表达,干细胞因子是一种重要的生殖和造血细胞因子。Steinmetz等人在2000年使用大鼠Sertoli细胞的原代培养物比较了GHRH-RP和GHRH 1处理后的作用时间和SCF刺激水平。生长激素释放激素从神经分泌神经末梢释放,并由下丘脑-垂体门系统携带至垂体前叶腺。它通过刺激生长激素释放激素受体2刺激GH分泌。在两栖性下丘脑中发现了一种新的Arg-Phe-NH 2(RFamide)肽。含有该肽的细胞体和末端分别位于视交叉上核和中位隆起。这种肽被进一步显示出具有相当大的生长激素(GH) -释放活性在体外和体内,并因此命名为青蛙GH释放肽(FGRP)3。
结构特征
GH或生长激素是在垂体1的前部产生的191个氨基酸的蛋白质激素。人胎盘催乳素(hPL),生长激素和催乳激素(PRL)构成生长激素家族。它们都具有约200个氨基酸,2个二硫键,并且没有糖基化作用。尽管每种都具有特殊的受体并具有独特的活性特征,但它们都具有促进生长和生乳的活性。成熟的GH(22,000道尔顿)在嗜酸性垂体生长激素中合成为单个多肽链4,5。编码fGRP前体多肽的cDNA的分子克隆表明,它编码fGRP及其推定的基因相关肽(fGRP-RP-1,-RP-2和-RP-3)。随后,作者将这些推定的fGRP-RPs鉴定为成熟肽,并分析了它们的促生理活性。在体外和体内,只有fGRP-RP-2刺激GH和催乳激素(PRL)的释放。因此,除了fGRP,fGRP-RP-2还充当青蛙垂体的下丘脑因子,刺激GH和PRL 3的释放。
行动方式
GH或生长激素的释放受两种激素的相反作用控制:生长激素释放激素,它既刺激生长激素的合成又分泌;和生长抑素,抑制其释放。GH在人体几乎每个器官和系统的几个复杂生理过程的调节中都起着重要作用。它促进软组织,软骨和骨骼的生长;增加蛋白质,RNA和DNA的合成;减少碳水化合物的利用并增加脂肪的利用。GH通过对增殖性软骨细胞或干细胞软骨细胞的分化产生刺激作用来促进骨生长。GH的许多作用是由肝脏和其他组织分泌的胰岛素样生长因子1(IGF-1)介导的。生长激素的过量产生会导致青年人的巨人症和成年人的肢端肥大症。儿童缺乏激素会导致侏儒症4、5。在人类中,生长激素促进糖异生,因此是高血糖的。它促进细胞摄取氨基酸,结果是GH疗法使生物体处于正氮平衡,这与成长中的儿童相似。
功能
生长激素释放激素 还直接促进慢波睡眠。正常的产后生长,骨骼生长,对蛋白质,碳水化合物和脂质代谢的调节作用都需要生长激素1。
遗传缺陷, 与GH相关的遗传缺陷很多。缺乏GH的矮人缺乏合成或分泌GH的能力,这些矮个子的个体对GH治疗反应良好。侏儒缺乏对GH的IGF-1反应,但缺乏其代谢作用。因此,在侏儒症中,缺乏症本质上是后受体。
肢端肥大症, 这些个体的缺陷显然与无法通过产生IGF-1对GH产生反应有关。长骨的骨closure闭合前产生过量的GH导致巨人症,而骨epi闭合后GH过量时,骨的生长导致肢端肥大症的特征5。
脂肪分解,生长激素是脂肪分解,诱导组织脂类的分解,从而提供用于支持通过增加的氨基酸摄取引起的刺激蛋白质合成的能源供应。
参考
1. Steinmetz R, Lazzaro N, Rothrock JK, Pescovitz OH (2000). Effects of growth hormone-releasing hormone-related peptide on stem cell factor expression in cultured rat sertoli cells. Journal Endocrine, 12(3):323-327.
2. Obál F, Krueger J (2001). The somatotropic axis and sleep. Rev Neurol., 157(11):12-15.
3. Ukena K, Koda A, Yamamoto K, Iwakoshi-Ukena E, Minakata H, Kikuyama S, Tsutsui K (2006). Structures and diverse functions of frog growth hormone-releasing peptide (fGRP) and its related peptides (fGRP-RPs): a review. Journal of Experimental Zoology Part A: Comparative Experimental Biology, 305(9):815-821.
4. Englander EW, Gomez GA, Greeley GH Jr (2004). Alterations in stomach ghrelin production and in ghrelin-induced growth hormone secretion in the aged rat. Mech. Ageing Dev., 125(12):871-875.
5. Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R (2000). The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J. Anat., 197(4):575-585.
Definition
Insulin-like growth factors (IGF)-1 and IGF-2 are ubiquitously expressed peptides with sequence homology to insulin.
Related Peptides
IGFs interacts with a specific receptor on the cell membrane, namely, the IGF-I receptor (IGF-IR), and the interaction is regulated by a group of specific binding proteins. All of these molecules are considered to be members of the IGF family, which includes the polypeptide ligands IGF-I and IGF-II, two types of cell membrane receptors (i.e., IGF-IR and IGF-IIR), and six IGF-binding proteins (i.e., IGFBP-1 through IGFBP-6).
Structural Characteristics
IGFs
IGF-I and IGF-II are single-chain polypeptides. The two molecules have 62% homology in their amino acid sequences. The molecules share additional structural similarities, and their structures resemble the structure of proinsulin1. IGF I consists of 70 amino acid residues, IGF I1 of 67, grouped into domains A and B (similar to insulin), C (analogous to the connecting peptide of proinsulin) and D (not present in insulins). The three intrachain disulfide bridges in IGF 1 and I1 have shown to be located in analogous positions to those in (pro) insulin1.
IGFBPs
The primary structures of mammalian IGFBPs appear to contain three distinct domains of roughly equivalent sizes: the conserved N-terminal domain, the highly variable midregion, and the conserved C-terminal domain.N-terminal domain contains 80–93 amino acid residues after the signal. Ten to 12 of the 16–20 cysteines found in the prepeptides are located within this domain. In IGFBP-1 to -5, these 12 cysteines are fully conserved, whereas in IGFBP-6, 10 of the 12 cysteines are invariant2.Midregion ranging in size from 55 amino acid residues to 95 amino acids separates the N-terminal domain from the C-terminal domain. The amino acid sequence for each midsegment appears to be unique to the protein. C-terminal region are highly conserved and, 6 cysteines of the total 16–20 cysteines are found in the C terminus and are strictly conserved2.
IGF Receptors
Both IGF-IR and IGF-IIR are glycoproteins and are located on the cell membrane. IGF-IR is a tetramer of two identical a-subunits and two identical ß-subunits. Structurally, IGF-IR resembles the insulin receptor, and there is 60% homology between them. IGF-IIR is monomeric. Three ligand-binding regions are found in the extracellular domain of the receptor, one for IGF-II binding and two for proteins containing mannose-6-phosphate (M6P), including renin, proliferin, thyroglobulin, and the latent form of (TGF)-ß transforming growth factor2.
Mode of Action
Binding of IGFs to IGF-IR activates the receptor's tyrosine kinase activity, which triggers a cascade of reactions. Two distinct signal transduction pathways have been identified for IGF-IR. One pathway activates Ras protein, Raf protein, and mitogen-activated protein kinase, and the other pathway involves phosphoinositol-3-kinase. IGF-IR is involved in cell transformation. In vitro experiments have shown that removal of IGF-IR from the cell membrane by eliminating the IGF-IR gene, by suppressing its expression, or by inhibiting its function can abolish cell transformation3. IGFBPs have multiple and complex functions. IGFBPs are able to inhibit or to enhance the action of IGFs, resulting in either suppression or stimulation of cell proliferation. These opposing effects of IGFBPs on IGFs are determined by the molecular structures of the binding proteins. When binding to IGFs, IGFBPs play three major roles: 1) transporting IGFs, 2) protecting IGFs from degradation, and 3) regulating the interaction between IGFs and IGF-IR. Normally, IGFBPs have higher binding affinity to IGFs than does IGF-IR; therefore, binding of IGFBPs to IGFs blocks the interaction between IGFs and IGF-IR and suppresses IGF action. However, binding of IGFBPs to IGFs also protects IGFs from proteolytic degradation, and that protection can enhance the action of IGFs by increasing their bioavailability in local tissue3.
Functions
Direct Involvement in Cancer - IGF-I and IGF-II are strong mitogens for a wide variety of cancer cell lines. Animal experiments indicate that overexpression of IGF-I increase the likelihood of tumor development in certain tissues. The effects of IGFs on cancer cells are mediated through IGF-IR. Eliminating IGF-IR from the cell membrane, blocking the interaction of IGFs with IGF-IR, or interrupting the signal transduction pathway of IGF-IR can abolish the mitogenic action of IGFs on cancer cells. IGF-IR is overexpressed in certain cancers, and its overexpression is associated with aggressive tumors. A recent study indicates that the insulin receptor is involved in mediating the actions of IGF-II on breast cancer. Cancer cells with a strong tendency to metastasize have higher expression of IGF-II and IGF-IR than those with a low ability to do so.In cancer, IGFBPs regulate the action of IGFs. In most situations, the binding proteins suppress the mitogenic action of IGFs and promote apoptosis. It has been shown that IGFBP-3 inhibited breast cancer cell growth without interacting with IGFs4.
IGF I protects and rescues hippocampal neurons against ß-amyloid- and human amylin-induced toxicity - Insulin-like growth factors (IGF-I and IGF-II) are well known trophic factors and their specific receptors are uniquely distributed throughout the brain, being especially concentrated in the hippocampal formation. IGFs possess neurotrophic activities in the hippocampus, an area severely affected in Alzheimer disease. There is evidence that ß-amyloid (aß)-derived peptides likely play an important role in the neurodegenerative process observed in Alzheimer disease, it has been shown that IGFs can be neuroprotective to hippocampal neurons against toxicity induced by amyloidogenic derivatives5.
Reference:
1. Daughaday WH, Rotwein P (1989). Insulin-like growth factors I and II - Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr. Rev, 10:68–91.
2. Jones JI, Clemmons DR (1995). Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev., 16:3–34.
3. Clemmons DR (1997). Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev, 8:45–62.
4. Yu H, Rohan T (2000). Role of the Insulin-Like Growth Factor Family in Cancer Development and Progression. Journal of the National Cancer Institute., 92 (18):1472-1489.
5. Doré S, Kar S, Quirion R (1997). Insulin-like growth factor I protects and rescues hippocampal neurons against ß-amyloid- and human amylin-induced toxicity. Proc. Natl. Acad. Sci, 94:4772–4777.
| DOI | 名称 | |
|---|---|---|
| 10.1186/1479-5876-10-224 | Human conditions of insulin-like growth factor-I (IGF-I) deficiency | 下载 |





