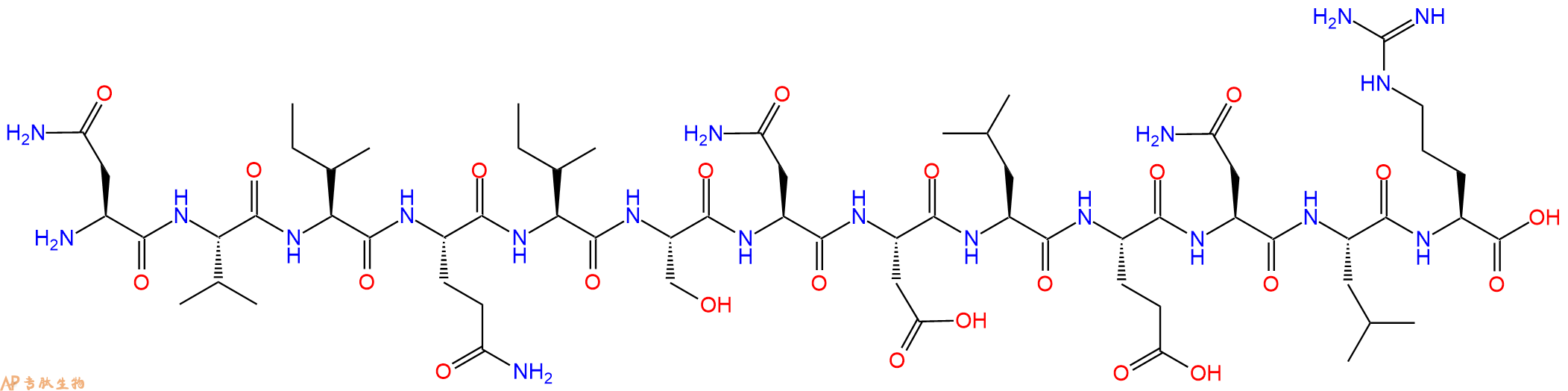
人体瘦素的 93-105 氨基酸片段。Leptin 是一种 167 个残基的肽激素,主要由脂肪细胞产生,并在中枢神经系统中起作用,主要协调代谢对禁食的适应。
编号:177021
CAS号:200436-43-7
单字母:H2N-NVIQISNDLENLR-OH
| 编号: | 177021 |
| 中文名称: | Leptin(93-105), human |
| 英文名: | Leptin(93-105), human |
| CAS号: | 200436-43-7 |
| 单字母: | H2N-NVIQISNDLENLR-OH |
| 三字母: | H2N N端氨基 -Asn天冬酰胺 -Val缬氨酸 -Ile异亮氨酸 -Gln谷氨酰胺 -Ile异亮氨酸 -Ser丝氨酸 -Asn天冬酰胺 -Asp天冬氨酸 -Leu亮氨酸 -Glu谷氨酸 -Asn天冬酰胺 -Leu亮氨酸 -Arg精氨酸 -OHC端羧基 |
| 氨基酸个数: | 13 |
| 分子式: | C64H110N20O23 |
| 平均分子量: | 1527.68 |
| 精确分子量: | 1526.81 |
| 等电点(PI): | 6.46 |
| pH=7.0时的净电荷数: | -0.02 |
| 平均亲水性: | 0.10769230769231 |
| 疏水性值: | -0.42 |
| 外观与性状: | 白色粉末状固体 |
| 消光系数: | - |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 纯度: | 95%、98% |
| 盐体系: | 可选TFA、HAc、HCl或其它 |
| 生成周期: | 2-3周 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 瘦素(Leptin) 肥胖研究 |
Leptin (93-105) 是人体瘦素的 93-105 氨基酸片段。Leptin 是一种 167 个残基的肽激素,主要由脂肪细胞产生,并在中枢神经系统中起作用,主要协调代谢对禁食的适应。
Leptin (93-105), human, is the amino acids 93 to 105 fragment of human leptin. Leptin is a 167-residue peptide hormone mainly produced by adipocytes and acts in the central nervous system to primarily coordinate the metabolic adaptations to fasting[1][2].
Definition
Leptin is a 16 kDa protein hormone originally discovered in adipose tissue where it acts as a satiety factor in signalling whole body energy balance.
Discovery
In 1950, at Jackson Lab the effects of leptin were observed while studying obese mice with random mutation 1. The obese yellow mice were attaining weights up to 75 or 80 grams. Later leptin was discovered in 1994 by Jeffrey M. Friedman et al., through the study of such mice. Mutation of ob (obese gene) resulted in profound obesity and type II diabetes as part of a syndrome that resembles morbid obesity in humans 2.
Structural Characteristics
Leptin of humans has 146 amino acid sequence containing one disulphide bond. Its molecular weight is around 16 kDa. Leptin has 67% sequence identity among diverse species. Leptin is a four-helix bundle with one very short strand segment and two relatively long interconnected loops. This is consistent with a classification as a cytokine four-helix bundle 3.
Mode of Action
Leptin acts via specific receptors (Ob-R), of which six isoforms are at present recognized (from Ob-Ra to Ob-Rf). Ob-Rb is the only isoform able to activate JAK-STAT and MAPK signaling cascades. A large body of evidence suggests that leptin and its receptors are involved in prostate physiology and pathophysiology in humans. Ob-R in rat is involved in the autocrine-paracrine functional regulation of the epithelial cells of adult rat seminal vesicles and prostate 4. Once leptin has bound to the Ob-Rb receptor, it activates the stat3, which is phosphorylated and travels to the nucleus to, presumably, effect changes in gene expression. One of the main effects on gene expression is the down-regulation of the expression of endocannabinoids, responsible for increasing appetite. In response to leptin, receptor neurons have been shown to remodel themselves, changing the number and types of synapses that fire onto them. Mutation of ob results in profound obesity and type II diabetes as part of a syndrome that resembles morbid obesity in humans. The ob gene product may function as part of a signalling pathway from adipose tissue that acts to regulate the size of the body fat depot 5.
Functions
Adiposity signal, Leptin is an adiposity hormone that modulates the activity of multiple hypothalamic signaling pathways involved in the control of food intake. Administration of leptin or one of its downstream mediators, neuropeptide Y (NPY), could affect food intake by modulating the brain stem neurophysiological response to ascending meal-related feedback signals in the nucleus of the solitary tract (NTS) in anesthetized male Long-Evans rats 6.
Appetite control, leptin binds to NPY neurons in the arculate nucleus, in such a way that decreases the activity of these neurons. Leptin signals to the brain that the body has had enough to eat, or satiety. A very small group of humans possess homozygous mutations for the leptin gene which leads to a constant desire for food, resulting in severe obesity.
Modulation of T cell activity, the role of Leptin/Leptin receptors in modulation of T cell activity in immune system was shown in experimentation with mice. Leptin/leptin receptor pathway is involved in the modulation of the regulatory immune response in atherosclerosis, and alteration in regulatory immunity may predispose obese individuals to atherosclerosis 7.
Lung surfactant activity, in fetal lung leptin is induced in the alveolar interstitial fibroblasts ("lipofibroblasts") by the action of PTHrP secreted by formative alveolar epithelium (endoderm) under moderate stretch. The leptin from the mesenchyme in turn acts back on the epithelium at the leptin receptor carried in the alveolar type II pneumocytes and induces surfactant expression which is one of the main functions of these type II pneumocytes 8.
Reproduction, it appears that leptin and estradiol interact coordinately in a concentration-dependent manner to control IVF outcome. In mice, leptin is also required for male and female fertility. In mammals, ovulatory cycles in females are linked to energy balance (positive or negative depending on whether a female is losing or gaining weight) and energy flux (how much energy is consumed and expended) much more than energy status (fat levels) 9.
References
1. Ingalls AM, Dickie MM, Snell GD (1950). Obese, a new mutation in the house mouse. J. Hered., 41(12):317-318.
2. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994). Positional cloning of the mouse obese gene and its human homologue. Nature, 372(6505):425–432.
3. Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM, Schoner BE, Smith DP, Zhang XY, Wery JP, Schevitz RW (1997). Crystal structure of the obese protein leptin-E100. Nature, 387:206-209..
4. Malendowicz W, Rucinski M, Macchi C, Spinazzi R, Ziolkowska A, Nussdorfer GG, Kwias Z (2006). Leptin and leptin receptors in the prostate and seminal vesicles of the adult rat. Int. J. Mol. Med., 18(4):615–618.
5. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994). Positional cloning of the mouse obese gene and its human homologue. Nature, 372(6505):425–432.
6. Williams KW, Scott MM, Elmquist JK (2009). From observation to experimentation: leptin action in the mediobasal hypothalamus. Am. J. Clin. Nutr., 89(3):985–990.
7. Taleb S, Herbin O, Ait-Oufella H, Verreth W, Gourdy P, Barateau V, Merval R, Esposito B, Clément K, Holvoet P, Tedgui A, Mallat Z (2007). Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis.. Arterioscler Thromb Vasc Biol., 27(12):2691–2698.
8. Torday JS, Rehan VK (2006). Up-regulation of fetal rat lung parathyroid hormone-related protein gene regulatory network down-regulates the Sonic Hedgehog/Wnt/betacatenin gene regulatory network. Pediatr Res., 60(4):382–388.
9. Anifandis G, Koutselini E, Louridas K, Liakopoulos V, Leivaditis K, Mantzavinos T, Sioutopoulou D, Vamvakopoulos N (2005). Estradiol and leptin as conditional prognostic IVF markers. Reproduction, 129(4):531–534.
Andreoli MF, Donato J, Cakir I, Perello M. Leptin resensitisation: a reversion of leptin-resistant states. J Endocrinol. 2019;241(3):R81-R96. : https://pubmed.ncbi.nlm.nih.gov/30959481/





