CTAP已被发现是一种选择性的μ-阿片受体拮抗剂。
编号:184854
CAS号:103429-32-9
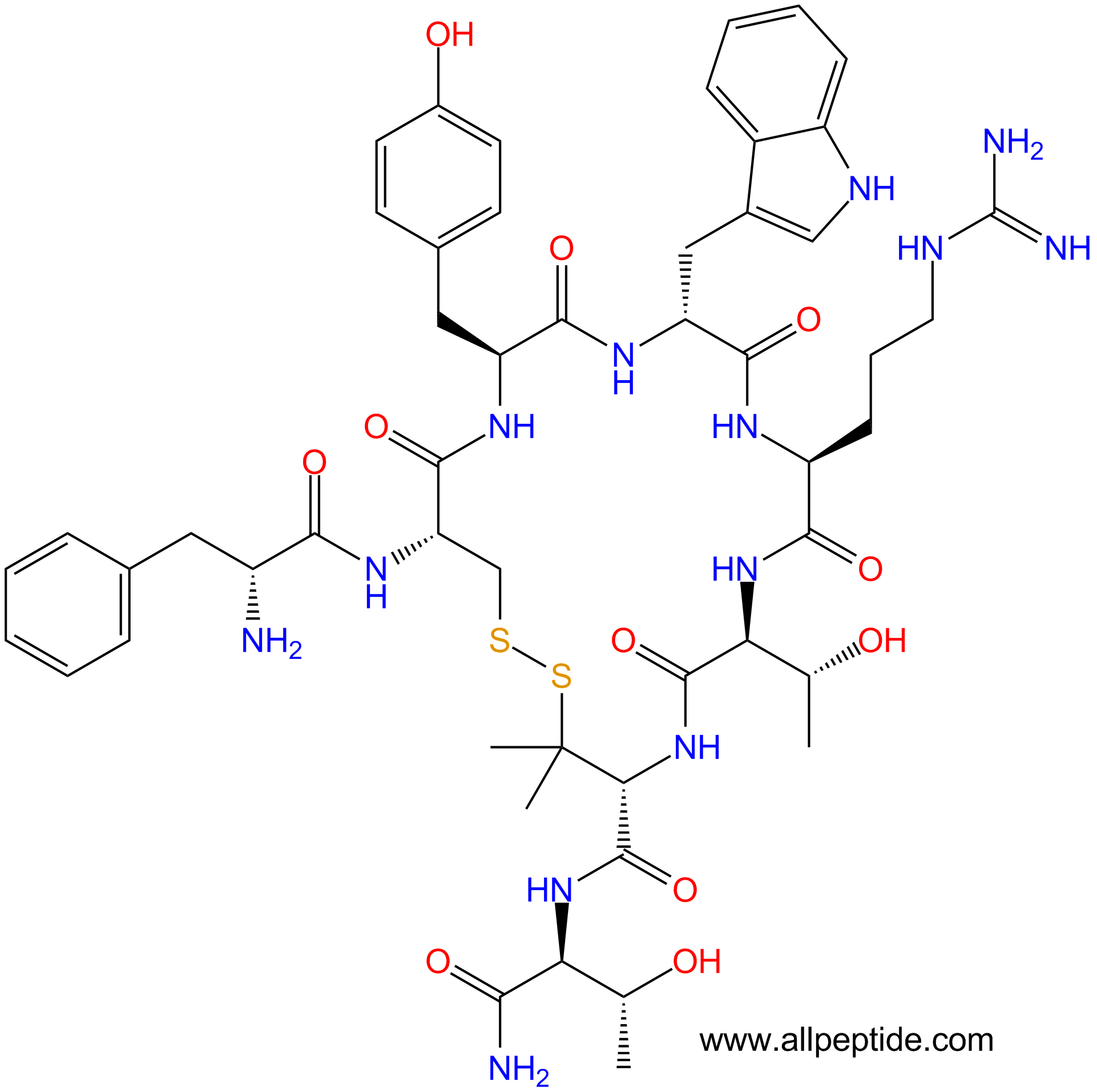
单字母:H2N-fCYwRT-Pen-T-NH2(Disulfide Bridge:C2-Pen7)
| 编号: | 184854 |
| 中文名称: | 选择性的μ-阿片受体拮抗剂:CTAP |
| 英文名: | CTAP |
| CAS号: | 103429-32-9 |
| 单字母: | H2N-fCYwRT-Pen-T-NH2(Disulfide Bridge:C2-Pen7) |
| 三字母: | H2N-DPhe-Cys-Tyr-DTrp-Arg-Thr-Pen-Thr-NH2(Disulfide Bridge:Cys2-Pen7) |
| 氨基酸个数: | 8 |
| 分子式: | C51H69N13O11S2 |
| 平均分子量: | 1104.3 |
| 精确分子量: | 1103.47 |
| 等电点(PI): | - |
| pH=7.0时的净电荷数: | 3.94 |
| 平均亲水性: | -1.1571428571429 |
| 疏水性值: | -0.34 |
| 外观与性状: | 白色粉末状固体 |
| 消光系数: | 6990 |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 纯度: | 95%、98% |
| 盐体系: | 可选TFA、HAc、HCl或其它 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 二硫键环肽 脑啡肽(Enkephalins) 拮抗剂相关肽(Antagonist Peptide) |
CTAP已被发现是一种选择性的μ-阿片受体拮抗剂。
CTAP, an analog of CTOP, is a selective µ-opioid receptor antagonist.
CTAP是µ阿片受体(IC50=3.5 nM)相对于δ受体(IC50=4500 nM)的水溶性选择性拮抗剂。它是一种环状八肽,作为生长抑素受体的不良拮抗剂(IC50=14.3μM)。CTAP的效力至少是纳曲酮的10倍。
CTAP is a water-soluble and selective antagonist of the µ opioid receptor (IC50 = 3.5 nM) over the δ receptor (IC50 = 4,500 nM). It is a cyclic octapeptide which acts as a poor antagonist of the somatostatin receptor (IC50 = 14.3 μM). CTAP is at least 10-fold more potent than naltrexone.
二硫键广泛存在与蛋白结构中,对稳定蛋白结构具有非常重要的意义,二硫键一般是通过序列中的2个Cys的巯基,经氧化形成。
形成二硫键的方法很多:空气氧化法,DMSO氧化法,过氧化氢氧化法等。
二硫键的合成过程, 可以通过Ellman检测以及HPLC检测方法对其反应进程进行监测。
如果多肽中只含有1对Cys,那二硫键的形成是简单的。多肽经固相或液相合成,然后在pH8-9的溶液中进行氧化。
当需要形成2对或2对以上的二硫键时,合成过程则相对复杂。尽管二硫键的形成通常是在合成方案的最后阶段完成,但有时引入预先形成的二硫化物是有利于连合或延长肽链的。通常采用的巯基保护基有trt, Acm, Mmt, tBu, Bzl, Mob, Tmob等多种基团。我们分别列出两种以2-Cl树脂和Rink树脂为载体合成的多肽上多对二硫键形成路线:
二硫键反应条件选择
二硫键即为蛋白质或多肽分子中两个不同位点Cys的巯基(-SH)被氧化形成的S-S共价键。 一条肽链上不同位置的氨基酸之间形成的二硫键,可以将肽链折叠成特定的空间结构。多肽分 子通常分子量较大,空间结构复杂,结构中形成二硫键时要求两个半胱氨酸在空间距离上接近。 此外,多肽结构中还原态的巯基化学性质活泼,容易发生其他的副反应,而且肽链上其他侧链 也可能会发生一系列修饰,因此,肽链进行修饰所选取的氧化剂和氧化条件是反应的关键因素, 反应机理也比较复杂,既可能是自由基反应,也可能是离子反应。
反应条件有多种选择,比如空气氧化,DMSO氧化等温和的氧化过程,也可以采用H2O2,I2, 汞盐等激烈的反应条件。
空气氧化法: 空气氧化法形成二硫键是多肽合成中最经典的方法,通常是将巯基处于还原态的多肽溶于水中,在近中性或弱碱性条件下(PH值6.5-10),反应24小时以上。为了降低分子之间二硫键形成的可能,该方法通常需要在低浓度条件下进行。
碘氧化法:将多肽溶于25%的甲醇水溶液或30%的醋酸水溶液中,逐滴滴加10-15mol/L的碘进行氧化,反应15-40min。当肽链中含有对碘比较敏感的Tyr、Trp、Met和His的残基时,氧化条件要控制的更精确,氧化完后,立即加入维生素C或硫代硫酸钠除去过量的碘。 当序列中有两对或多对二硫键需要成环时,通常有两种情况:
自然随机成环: 序列中的Cys之间随机成环,与一对二硫键成环条件相似;
定点成环: 定点成环即序列中的Cys按照设计要求形成二硫键,反应过程相对复杂。在 固相合成多肽之前,需要提前设计几对二硫键形成的顺序和方法路线,选择不同的侧链 巯基保护基,利用其性质差异,分步氧化形成两对或多对二硫键。 通常采用的巯基保护 基有trt, Acm, Mmt, tBu, Bzl, Mob, Tmob等多种基团。
脑啡肽和前脑啡肽
定义
脑啡肽是在大脑和内分泌组织中高水平发现的阿片类肽。新出现的含有脑啡肽的肽的主要种类似乎是完整的前体,前脑啡肽1。
相关肽
阿片肽构成了一大类小蛋白,它们与鸦片生物碱,吗啡和海洛因相似,能与细胞膜受体相互作用。阿片生物碱衍生物广泛用于镇痛和麻醉。最初的阿片肽家族是脑啡肽,强啡肽和内啡肽。在心脏中已经发现了来自这三个阿片肽家族的代表性肽。已克隆并测序了三种不同的阿片受体:mu(µ),delta(d)和kappa(?)2。
发现
Kosterlitz和Hughes在1975年发现了脑啡肽和内啡肽3。
结构特征
蛋氨酸-脑啡肽(Met-Enk)的氨基酸序列为酪氨酸-甘氨酸-甘氨酸-苯丙氨酸-蛋氨酸。前脑啡肽序列包含五肽Met-Enk的四个拷贝,leu-脑啡肽之一和Met- enk的两个扩展形式(Met- enk -arg 6 -phe 7和met- enk -arg 6 -gly 7 -leu 8)。成对的碱性氨基酸标记这些小肽从前体裂解。原脑啡肽由称为原激素转化酶的内蛋白水解酶加工,该酶在二元氨基酸位点识别并切割。最初的前脑啡肽处理开始于传输到高尔基体网络之前,并且过程很快。后续处理需要在高尔基体网络远端的酸性环境中进行。前脑啡肽对肽B具有快速裂解,而较慢的裂解产生其他中等大小的产物,其最终被裂解成五肽至八肽。在各种组织(肌肉,神经,内分泌)中发现的不同分子量的最终产物可能是由于切割序列的差异和加工所需的局部酶促条件2所致。
作用方式
肽Met-Enk和Leu-Enk(阿片受体的内源性配体)起神经调节剂或神经递质的作用。脑啡肽在哺乳动物脑中最显着的作用是神经元放电速率的降低,并且已经表明这些肽是抑制性递质。脑啡肽抑制或增强了中枢神经元对几种假定的递质的反应,表明突触后的作用。还显示脑啡肽抑制K +诱导的去甲肾上腺素,多巴胺和乙酰胆碱从大鼠脑片的释放,表明突触前的作用。脑啡肽抑制豚鼠回肠中的肌间神经元的发射。这种抑制可能是由于脑啡肽的直接突触后作用导致神经元膜超极化4。。为了实现其生物学功能,必须将脑啡肽从水相转运至其膜结合受体蛋白的富含脂质的环境。现已知道,Met-enk通过三种主要的亚型受体起作用,分别称为μ,d和β。-受体。虽然前两个受体亚型介导了Met-enk的经典阿片样物质作用,但据报道β受体参与了该肽的非阿片样物质作用,即对细胞生长的抑制作用5。
功能
前脑啡肽是神经肽的前体,在神经内分泌和神经系统中具有多种功能。激活后,发现T辅助淋巴细胞表达高水平的前脑啡肽mRNA,并分泌大量的Met-Enk神经肽,这可能表明了免疫系统和神经系统相互作用的轴6。脑啡肽引起抗伤害感受和增强的吗啡镇痛作用,但它们也阻碍了耐受性和身体依赖性的发展。除了其中枢和外周镇痛作用外,阿片类药物还可以调节免疫活性和细胞增殖。此外,众所周知,它们在不同的生理过程中具有重要作用,例如细胞分化和再生,炎症,癌症和血管生成以及镇痛作用5。
参考
1、Fleminger G, Lahm HW, Udenfriend S (1984).Changes in rat adrenal catecholamines and proenkephalin metabolism after denervation. PNAS., 81(11):3587-3590.
2、Barbara A. Barron. 2000. Cardiac Opioids. Proceedings of the Society for Experimental Biology and Medicine, 224:1-7.
2、Fratta W, Yang HY, Hong J, Costa E (1977). Stability of Met-enkephalin content in brain structures of morphine-dependent or foot shock-stressed rats. Nature, 268(5619):452-453.
4、Wouters W, Den Bercken JV (1979). Hyperpolarisation and depression of slow synaptic inhibition by enkephalin in frog sympathetic ganglion. Nature, 277:53-54.
5、Tsanova A, Dacheva D, Penchev V, Georgiev G, Pajpanova T, Golovinski E, Lalchev Z (2009). Comparative study of the interaction between synthetic methionine-enkephalin and monolayers of zwitterionic and negatively 6、charged phospholipids. Biotechnol & Biotechnol., 23:463-466.
7、Rattner A, Korner M, Rosen H, Baeuerle PA, Citri Y (1991). Nuclear factor Kappa B activates proenkephalin transcription in T lymphocytes. Molecular and Cellular Biology, 11(2):1017-1022.
Enkephalins and Proenkephalins
Definition
Enkephalins are opioid peptides that are found at high levels in the brain and endocrine tissues. The major species of newly appearing enkephalin-containing peptide appears to be the intact precursor, proenkephalin 1.
Related Peptides
Opioid peptides constitute a large group of small proteins that interact with cell membrane receptors similarly to opiate alkaloids, morphine and heroin. Opiate alkaloid derivatives are extensively used for analgesia and anesthesia. The original opioid peptide families are enkephalins, dynorphins, and endorphins. Representative peptides from these three opioid peptide families have been found in the heart. Three different opiate receptors have been cloned and sequenced: mu (µ), delta (d), and kappa (?) 2.
Discovery
Kosterlitz and Hughes discovered enkephalins and endorphins in 1975 3.
Structural Characteristics
The amino acid sequence of methionine-enkephalin (Met-Enk) is tyrosine-glycine-glycine-phenylalanine-methionine. The proenkephalin sequence contains four copies of the pentapeptide Met-Enk, one of leu-enkephalin, and two extended forms of Met-enk (Met-enk-arg6-phe7 and met-enk-arg6-gly7-leu8). Pairs of basic amino acids mark these small peptides for cleavage from the precursor. Proenkephalin is processed by endoproteolytic enyzmes termed prohormone convertases, which recognize and cleave at dibasic amino acid sites. Initial proenkephalin processing starts before transport to the golgi network and are rapid. Later processing requires an acidic environment distal to the golgi network. Proenkephalin has a fast cleavage to peptide B, and slower cleavages yield other intermediate sized products that are cleaved ultimately to the penta to octapeptides. The different molecular-weight end products found in diverse tissues (muscle, neural, endocrine) may be due to variations in the cleavage sequence and local enzymatic conditions for processing 2.
Mode of Action
Pentapeptides Met-Enk and Leu-Enk, the endogenous ligands for the opiate receptor, function as neuromodulators or neurotransmitters. The most prominent action of enkephalins in the mammalian brain is depression of neuronal firing rate and it has been suggested that these peptides are inhibitory transmitters. The response of central neurones to several putative transmitter substances is depressed or enhanced by enkephalins, suggesting a postsynaptic action. It has also been shown that enkephalins suppress the K+-induced release of noradrenaline, dopamine and acetylcholine from rat brain slices, indicating a presynaptic effect. The firing of myenteric neurones in the guinea-pig ileum is inhibited by enkephalins. This inhibition is probably due to a direct postsynaptic action of the enkephalins resulting in a hyperpolarisation of the neuronal membrane 4. To achieve their biological function, enkephalins must be transported from an aqueous phase to the lipid-rich environment of their membrane bound receptor proteins. It is now known that Met-enk acts via three main subtypes of receptors referred to as µ, d and ? - receptors. While the first two receptor subtypes mediate the classic opioid effects of Met-enk, ?-receptors are reported to be involved in the non-opioid actions of the peptide, i.e. the inhibitory effect on the cell growth 5.
Functions
Proenkephalin is a precursor for neuropeptides with a variety of functions in the neuroendocrine and nervous systems. Upon activation, T-helper lymphocytes were found to express high levels of proenkephalin mRNA and to secrete large amounts of the Met-Enk neuropeptide, perhaps indicating an axis by which the immune and nervous systems interact 6. Enkephalins cause antinociception and potentiated morphine analgesia but they also block the development of tolerance and physical dependence. In addition to their central and peripheral antinociceptive function, opioids can modulate immune activity and cell proliferation. Moreover it is known that they have significant role in different physiological processes like cell differentiation and regeneration, inflammation, cancer and angiogenesis and analgesia effects 5.
References
Fleminger G, Lahm HW, Udenfriend S (1984).Changes in rat adrenal catecholamines and proenkephalin metabolism after denervation. PNAS., 81(11):3587-3590.
Barbara A. Barron. 2000. Cardiac Opioids. Proceedings of the Society for Experimental Biology and Medicine, 224:1-7.
Fratta W, Yang HY, Hong J, Costa E (1977). Stability of Met-enkephalin content in brain structures of morphine-dependent or foot shock-stressed rats. Nature, 268(5619):452-453.
Wouters W, Den Bercken JV (1979). Hyperpolarisation and depression of slow synaptic inhibition by enkephalin in frog sympathetic ganglion. Nature, 277:53-54.
Tsanova A, Dacheva D, Penchev V, Georgiev G, Pajpanova T, Golovinski E, Lalchev Z (2009). Comparative study of the interaction between synthetic methionine-enkephalin and monolayers of zwitterionic and negatively charged phospholipids. Biotechnol & Biotechnol., 23:463-466.
Rattner A, Korner M, Rosen H, Baeuerle PA, Citri Y (1991). Nuclear factor Kappa B activates proenkephalin transcription in T lymphocytes. Molecular and Cellular Biology, 11(2):1017-1022.
M.K.Mundey et al., Br. J. Pharmacol., 131, 893 (2000);
E.J.Bilsky et al., J. Pharmacol. Exp. Ther., 277, 484 (1996);





