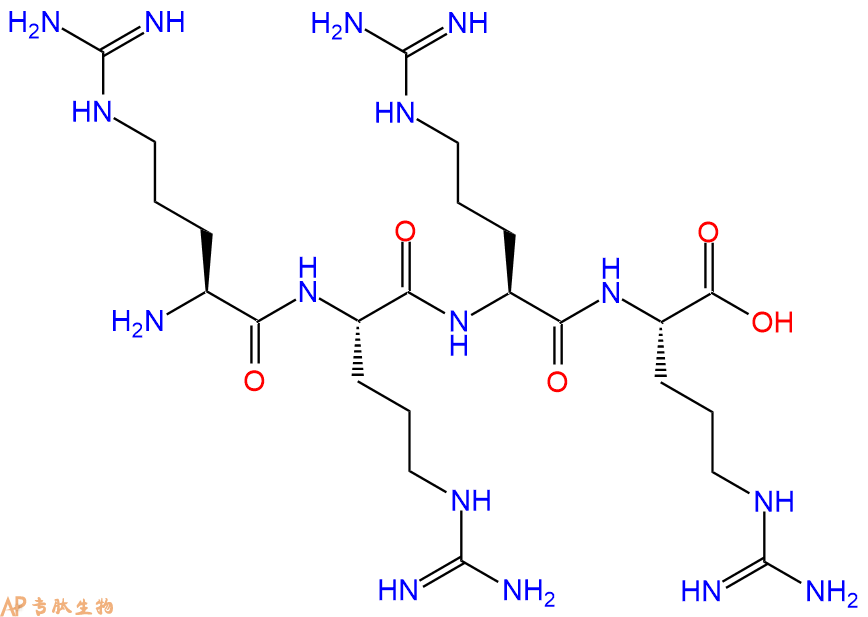
四精氨酸是 Fugere 等人测试的最短的寡精氨酸。并显示抑制前蛋白转化酶 5/6 和 7。
编号:111238
CAS号:26791-46-8
单字母:H2N-RRRR-OH
| 编号: | 111238 |
| 中文名称: | 四聚精氨酸、Arg4、R4、(Arg)4 |
| 英文名: | Cell penetrating peptide R4、Arg4、(Arg)4 |
| CAS号: | 26791-46-8 |
| 单字母: | H2N-RRRR-OH |
| 三字母: | H2N N端氨基 -Arg精氨酸 -Arg精氨酸 -Arg精氨酸 -Arg精氨酸 -OHC端羧基 |
| 氨基酸个数: | 4 |
| 分子式: | C24H50N16O5 |
| 平均分子量: | 642.76 |
| 精确分子量: | 642.41 |
| 等电点(PI): | - |
| pH=7.0时的净电荷数: | 4.97 |
| 平均亲水性: | 3 |
| 疏水性值: | -4.5 |
| 外观与性状: | 粉末状固体 |
| 消光系数: | - |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 纯度: | 95%、98% |
| 盐体系: | 可选TFA、HAc、HCl或其它 |
| 生成周期: | 2-3周 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 细胞穿膜肽(Cell permeable peptides, CPPs) 抑制剂相关肽(Inhibitor Peptide) |
四精氨酸是Fugere等人测试的最短的寡精氨酸,并显示出抑制前蛋白转化酶5/6和7。
Tetraarginine was the shortest oligoarginine tested by Fugere et al. and shown to inhibit proprotein convertases 5/6 and 7.
H-Arg-Arg-Arg-OH是鱼精蛋白分子中常见的重复单元之一。
H-Arg-Arg-Arg-Arg-OH is one of the commonly observed repeat units in protamine molecules.
细胞穿膜肽-说明
穿透细胞膜进入细胞内是许多作用靶点在细胞内的生物大分子发挥作用的先决条件,然而生物膜的生物屏障作用阻止了许多高分子物质进入细胞内,从而很大程度地限制了这些物质在治疗领域的应用。因此,如何引导这些物质穿透细胞膜是一个迫切需要解决的问题,目前介导生物大分子穿透细胞膜的方法主要包括细胞穿透肽(cell penetrating peptides,CPPs)、脂质体、腺病毒、纳米颗粒、影细胞等,而CPPs是一类以非受体依赖方式,非经典内吞方式直接穿过细胞膜进入细胞的多肽,它们的长度一般不超过30个氨基酸且富含碱性氨基酸,氨基酸序列通常带正电荷。
1型人免疫缺陷病毒转录激活因子TAT(human immunodeficiency virus-1 transcription activator, HIV-1 TAT)是第一个被发现的细胞穿透肽,它凭借一种无毒的、高效的方式进入细胞。
细胞穿透肽(cell penetrating peptides,CPPs)的一个重要特点是可以携带多种不同大小和性质的生物活性物质进入细胞,包括小分子化合物、染料、多肽、多肽核酸(peptide nucleo acid, PNA)、蛋白质、质粒DNA、siRNA、200nm的脂质体、噬菌体颗粒和超顺磁性粒子等,这一性质为其成为靶向药物的良好载体提供了可能。
CPPs作为载体的优势在于低毒性和无细胞类型的限制,尽管CPPs可输送不同类型的物质进入细胞,但其实际应用多集中于寡肽、蛋白质、寡聚核苷(oligonucleotides,ONs)或类似物的细胞转运。
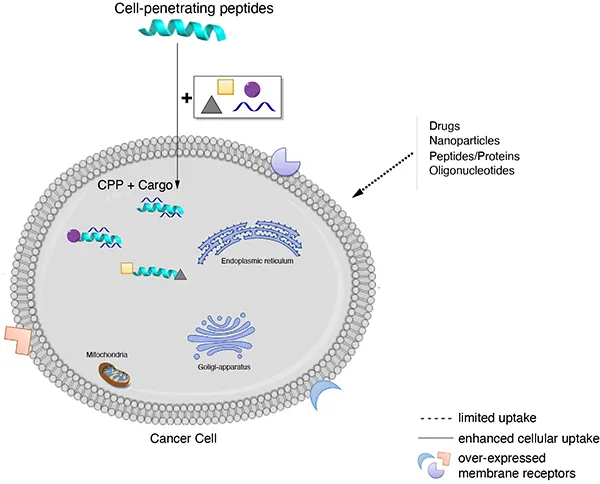
跨膜机理
不同的细胞穿透肽(CPP)跨膜机制不同,一个细胞穿透肽(CPP)的具体机制有赖于几个参数,如分子大小(携带物质)、温度、细胞类型和细胞内外的稳定性等。细胞穿透肽(CPP)进入细胞的具体机制目前还不清楚,比较流行的推测包括以下三种:
A: 倒置胶粒模型(inverted micelle model),CPPs通过细胞膜上磷脂分子的移动形成倒置胶粒结构,而进入胞浆。
B: 直接穿透,即孔隙结构模型 (pore formation model),CPPs在细胞膜上组成跨膜的孔隙结构而进入胞浆 。
C: 内吞方式进行细胞摄取。
来源: Cell-penetrating peptides and their therapeutic applications, Victoria Sebbage, BioscienceHorizons, Volume 2, Number 1, March 2009.
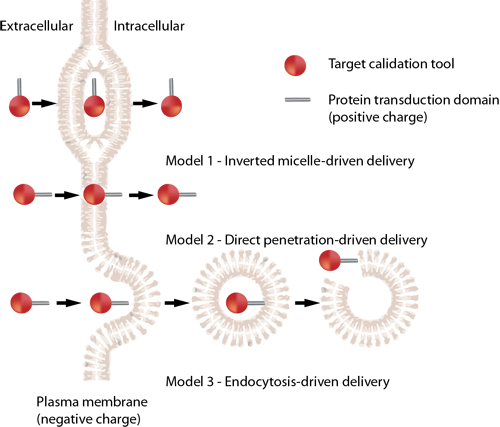
细胞穿透肽 HIV TAT
细胞穿透肽(如HIV TAT)可以以直接穿透和内吞两种方式进入细胞。HIV TAT或者简单的多聚精氨酸可被设计作为有效的药物载体,但CPP(如HIV TAT)是如何实现胞膜转运,目前仍不清楚。
简单的HIV TAT是如何促进象直接穿透和内吞作用的入胞机制的呢?来自Gerard Wong实验室的研究人员研究了在不同的条件下,HIV TAT是如何与细胞质膜、细胞骨架、特异的胞膜受体相互作用,从而诱导了多重转运途径。
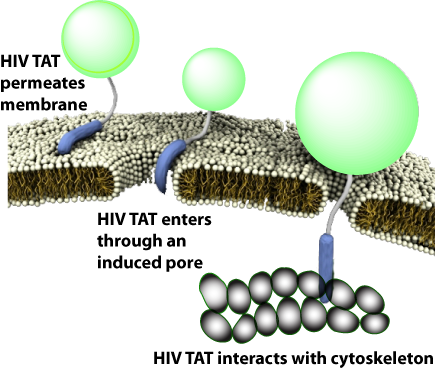
有趣的是,TAT在不同条件下可与同一序列发生多种不同的反应,因而与胞膜、细胞骨架、特异受体相互作用可产生多种转运途径。
CPP的跨膜机制与多肽序列存在很敏感的关系,如果在一个纯亲水性的CPP中增加一个疏水残基,就能彻底地改变其转运机制,例如,最简单的CPP原型-多聚精氨基(polyR),可以诱导细胞膜上形成跨膜的孔隙结构。疏水氨基酸通过插入胞膜来形成正曲率,精氨酸可同时形成正曲率和负曲率,赖氨酸只能沿一个方向形成负曲率,这就意味着在精氨酸与赖氨酸/疏水物之间存在补偿关系。
如果疏水性有助于形成负高斯曲率(Gaussian curvature),那为什么TAT肽中的疏水含量相对较低呢?其原因是CPPs都是利用尽可能少的疏水基去形成saddle-splay curvature。序列上的差异很可能只会在膜上诱导短暂的类似孔隙的跨膜结构,从而形成对CPP来说更短的孔隙寿命。由于CPP的氨基酸组成不同,TAT肽在有或无受体情况下都可以介导细胞内吞作用。
专肽生物提供各类细胞穿膜肽序列,部分由现货,例如TAT,R8,R4等,具体可咨询销售人员。
Definition
Cell permeable peptides (CPPs) are carriers with small peptide domains that can freely cross cell membranes. They are mainly used as carriers of proteins and nucleic acids into the cell1.
Discovery
The first CPP was discovered independently by two laboratories in 1988 when it was found that the trans-activating transcriptional activator (Tat) from Human Immunodeficiency Virus 1 (HIV-1) could be efficiently taken up from the surrounding media by numerous cell types in culture2.
Structural Characteristics
CPPs typically have an amino acid composition containing either a high relative abundance of positively charged, cationic amino acids such as lysine or arginine, or have sequences that contain an alternating pattern of polar/charged amino acids and non-polar, hydrophobic amino acids3. Some examples include: TAT peptide-YGRKKRRQRRR, lipid membrane translocating peptide-KKAAAVLLPVLLAAP and Antennapedia leader peptide-KKWKMRRNQFWVKVQRG.
Classification
Numerous CPPs have been identified to date and they belong to a wide variety of protein families. For example, some CPPs are amphipathic protein family members3.
Mode of action
CPPs enter the cell with their carrier by either of three mechanisms: Direct delivery that involves energy independent entry of the CPPs in to the cell4, endocytosis where the cells take up the CPPs by imbibing them with their cell membranes5 and translocation through the formation of transient structures which is yet to be understood6.
Functions
CPPs have found numerous applications in medicine as drug delivery agents in the treatment of different diseases including cancer, virus inhibitors, contrast agents for cell labeling a classical example is Green Fluorescent protein GFP, as MRI contrast agents, quantum dots7. TAT is very effective in delivering drugs in vitro and in vivo and so far a peptide that matches its efficiency has not been found7.
References
1. Wagstaff KM and David JA (2006). Protein Transduction: Cell Penetrating Peptides and Their Therapeutic Applications, Current Medicinal Chemistry, 13 (12), 1371-1387.
2. Feng S and Holland EC (1988). HIV-1 Tat trans-activation requires the loop sequence within Tar. Nature 334, 165–167.
3. Stewart KM, Horton KL, Kelley SO (2008). Cell-penetrating peptides as delivery vehicles for biology and medicine, Org Biomol Chem., 6(13), 2242-55.
4. Luo D, Saltzman WM (2000). Synthetic DNA delivery systems. Nat. Biotechnol, 18, 33-37.
5. Lundberg M., Wikstrom S and Johansson M (2003). Cell surface adherence and endocytosis of protein transduction domains, Mol. Ther., 8, 143–150.
6. Deshayes S, Gerbal-Chaloin S, Morris MC, Aldrian-Herrada G, Charnet P, Divita G (2004). On the mechanism of non-endosomial peptide-mediated cellular delivery of nucleic acids, Biochim. Biophys. Acta, 1667, 141–147.
7. Temsamani J and Vida P (2004). The use of cell-penetrating peptides for drug delivery, Drug Discovery Today, 9 (23), 1012-1019.
定义
酶是用于生化反应的非常有效的催化剂。它们通过提供较低活化能的替代反应途径来加快反应速度。酶作用于底物并产生产物。一些物质降低或什至停止酶的催化活性被称为抑制剂。
发现
1965年,Umezawa H分析了微生物产生的酶抑制剂,并分离出了抑制亮肽素和抗痛药的胰蛋白酶和木瓜蛋白酶,乳糜蛋白酶抑制的胰凝乳蛋白酶,胃蛋白酶抑制素抑制胃蛋白酶,泛磷酰胺抑制唾液酸酶,乌藤酮抑制酪氨酸羟化酶,多巴汀抑制多巴胺3-羟硫基嘧啶和多巴胺3-羟色胺酶酪氨酸羟化酶和多巴胺J3-羟化酶。最近,一种替代方法已应用于预测新的抑制剂:合理的药物设计使用酶活性位点的三维结构来预测哪些分子可能是抑制剂1。已经开发了用于识别酶抑制剂的基于计算机的方法,例如分子力学和分子对接。
结构特征
已经确定了许多抑制剂的晶体结构。已经确定了三种与凝血酶复合的高效且选择性的低分子量刚性肽醛醛抑制剂的晶体结构。这三种抑制剂全部在P3位置具有一个新的内酰胺部分,而对胰蛋白酶选择性最高的两种抑制剂在P1位置具有一个与S1特异性位点结合的胍基哌啶基。凝血酶的抑制动力学从慢到快变化,而对于胰蛋白酶,抑制的动力学在所有情况下都快。根据两步机理2中稳定过渡态络合物的缓慢形成来检验动力学。
埃米尔•菲舍尔(Emil Fischer)在1894年提出,酶和底物都具有特定的互补几何形状,彼此恰好契合。这称为“锁和钥匙”模型3。丹尼尔·科什兰(Daniel Koshland)提出了诱导拟合模型,其中底物和酶是相当灵活的结构,当底物与酶4相互作用时,活性位点通过与底物的相互作用不断重塑。
在众多生物活性肽的成熟过程中,需要由其谷氨酰胺(或谷氨酰胺)前体形成N末端焦谷氨酸(pGlu)。游离形式并与底物和三种咪唑衍生抑制剂结合的人QC的结构揭示了类似于两个锌外肽酶的α/β支架,但有多个插入和缺失,特别是在活性位点区域。几种活性位点突变酶的结构分析为针对QC相关疾病5的抑制剂的合理设计提供了结构基础。
作用方式
酶是催化化学反应的蛋白质。酶与底物相互作用并将其转化为产物。抑制剂的结合可以阻止底物进入酶的活性位点和/或阻止酶催化其反应。抑制剂的种类繁多,包括:非特异性,不可逆,可逆-竞争性和非竞争性。可逆抑制剂 以非共价相互作用(例如疏水相互作用,氢键和离子键)与酶结合。非特异性抑制方法包括最终使酶的蛋白质部分变性并因此不可逆的任何物理或化学变化。特定抑制剂 对单一酶发挥作用。大多数毒药通过特异性抑制酶发挥作用。竞争性抑制剂是任何与底物的化学结构和分子几何结构非常相似的化合物。抑制剂可以在活性位点与酶相互作用,但是没有反应发生。非竞争性抑制剂是与酶相互作用但通常不在活性位点相互作用的物质。非竞争性抑制剂的净作用是改变酶的形状,从而改变活性位点,从而使底物不再能与酶相互作用而产生反应。非竞争性抑制剂通常是可逆的。不可逆抑制剂与酶形成牢固的共价键。这些抑制剂可以在活性位点附近或附近起作用。
功能
工业应用中, 酶在商业上被广泛使用,例如在洗涤剂,食品和酿造工业中。蛋白酶用于“生物”洗衣粉中,以加速蛋白质在诸如血液和鸡蛋等污渍中的分解。商业上使用酶的问题包括:它们是水溶性的,这使得它们难以回收,并且一些产物可以抑制酶的活性(反馈抑制)。
药物分子,许多药物分子都是酶抑制剂,药用酶抑制剂通常以其特异性和效力为特征。高度的特异性和效力表明该药物具有较少的副作用和较低的毒性。酶抑制剂在自然界中发现,并且也作为药理学和生物化学的一部分进行设计和生产6。
天然毒物 通常是酶抑制剂,已进化为保护植物或动物免受天敌的侵害。这些天然毒素包括一些已知最剧毒的化合物。
神经气体( 例如二异丙基氟磷酸酯(DFP))通过与丝氨酸的羟基反应生成酯,从而抑制了乙酰胆碱酯酶的活性位点。
参考
1、Scapin G (2006). Structural biology and drug discovery. Curr. Pharm. Des., 12(17):2087–2097.
2、Krishnan R, Zhang E, Hakansson K, Arni RK, Tulinsky A, Lim-Wilby MS, Levy OE, Semple JE, Brunck TK (1998). Highly selective mechanism-based thrombin inhibitors: structures of thrombin and trypsin inhibited with rigid peptidyl aldehydes. Biochemistry, 37 (35):12094-12103.
3、Fischer E (1894). Einfluss der configuration auf die wirkung der enzyme. Ber. Dt. Chem. Ges., 27:2985–2993.
4、Koshland DE (1958). Application of a theory of enzyme specificity to protein synthesis. PNAS., 44 (2):98–104.
5、Huang KF, Liu YL, Cheng WJ, Ko TP, Wang AH (2005). Crystal structures of human glutaminyl cyclase, an enzyme responsible for protein N-terminal pyroglutamate formation. PNAS., 102(37):13117-13122.
6、Holmes CF, Maynes JT, Perreault KR, Dawson JF, James MN (2002). Molecular enzymology underlying regulation of protein phosphatase-1 by natural toxins. Curr Med Chem., 9(22):1981-1989.
Definition
Enzymes are very efficient catalysts for biochemical reactions. They speed up reactions by providing an alternative reaction pathway of lower activation energy. Enzyme acts on substrate and gives rise to a product. Some substances reduce or even stop the catalytic activities of enzymes are called inhibitors.
Discovery
In 1965, Umezawa H analysed enzyme inhibitors produced by microorganisms and isolated leupeptin and antipain inhibiting trypsin and papain, chymostatin inhibiting chymotrypsin, pepstatin inhibiting pepsin, panosialin inhibiting sialidases, oudenone inhibiting tyrosine hydroxylase, dopastin inhibiting dopamine 3-hydroxylase, aquayamycin and chrothiomycin inhibiting tyrosine hydroxylase and dopamine J3-hydroxylase . Recently, an alternative approach has been applied to predict new inhibitors: rational drug design uses the three-dimensional structure of an enzyme's active site to predict which molecules might be inhibitors 1. Computer-based methods for identifying inhibitor for an enzyme have been developed, such as molecular mechanics and molecular docking.
Structural Characteristics
The crystal structures of many inhibitors have been determined. The crystal structures of three highly potent and selective low-molecular weight rigid peptidyl aldehyde inhibitors complexed with thrombin have been determined. All the three inhibitors have a novel lactam moiety at the P3 position, while the two with greatest trypsin selectivity have a guanidinopiperidyl group at the P1 position that binds in the S1 specificity site. The kinetics of inhibition vary from slow to fast with thrombin and are fast in all cases with trypsin. The kinetics are examined in terms of the slow formation of a stable transition-state complex in a two-step mechanism 2.
Emil Fischer in 1894 suggested that both the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another.This is known as "the lock and key" model 3. Daniel Koshland suggested induced fit model where substrate and enzymes are rather flexible structures, the active site is continually reshaped by interactions with the substrate as the substrate interacts with the enzyme 4.
N-terminal pyroglutamate (pGlu) formation from its glutaminyl (or glutamyl) precursor is required in the maturation of numerous bioactive peptides. The structure of human QC in free form and bound to a substrate and three imidazole-derived inhibitors reveals an alpha/beta scaffold akin to that of two-zinc exopeptidases but with several insertions and deletions, particularly in the active-site region. The structural analyses of several active-site-mutant enzymes provide a structural basis for the rational design of inhibitors against QC-associated disorders 5.
Mode of Action
Enzymes are proteins that catalyze chemical reactions. Enzymes interact with substrate and convert them into products. Inhibitor binding can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. There are a variety of types of inhibitors including: nonspecific, irreversible, reversible - competitive and noncompetitive. Reversible inhibitors bind to enzymes with non-covalent interactions like hydrophobic interactions, hydrogen bonds, and ionic bonds. Non-specific methods of inhibition include any physical or chemical changes which ultimately denature the protein portion of the enzyme and are therefore irreversible. Specific Inhibitors exert their effects upon a single enzyme. Most poisons work by specific inhibition of enzymes. A competitive inhibitor is any compound which closely resembles the chemical structure and molecular geometry of the substrate. The inhibitor may interact with the enzyme at the active site, but no reaction takes place. A noncompetitive inhibitor is a substance that interacts with the enzyme, but usually not at the active site. The net effect of a non competitive inhibitor is to change the shape of the enzyme and thus the active site, so that the substrate can no longer interact with the enzyme to give a reaction. Non competitive inhibitors are usually reversible. Irreversible Inhibitors form strong covalent bonds with an enzyme. These inhibitors may act at, near, or remote from the active site .
Functions
Industrial application, enzymes are widely used commercially, for example in the detergent, food and brewing industries. Protease enzymes are used in 'biological' washing powders to speed up the breakdown of proteins in stains like blood and egg. Problems using enzymes commercially include: they are water soluble which makes them hard to recover and some products can inhibit the enzyme activity (feedback inhibition) .
Drug molecules, many drug molecules are enzyme inhibitors and a medicinal enzyme inhibitor is usually characterized by its specificity and its potency. A high specificity and potency suggests that a drug will have fewer side effects and less toxic. Enzyme inhibitors are found in nature and are also designed and produced as part of pharmacology and biochemistry 6.
Natural poisons are often enzyme inhibitors that have evolved to defend a plant or animal against predators. These natural toxins include some of the most poisonous compounds known.
Nerve gases such as diisopropylfluorophosphate (DFP) inhibit the active site of acetylcholine esterase by reacting with the hydroxyl group of serine to make an ester.
References
Scapin G (2006). Structural biology and drug discovery. Curr. Pharm. Des., 12(17):2087–2097.
Krishnan R, Zhang E, Hakansson K, Arni RK, Tulinsky A, Lim-Wilby MS, Levy OE, Semple JE, Brunck TK (1998). Highly selective mechanism-based thrombin inhibitors: structures of thrombin and trypsin inhibited with rigid peptidyl aldehydes. Biochemistry, 37 (35):12094-12103.
Fischer E (1894). Einfluss der configuration auf die wirkung der enzyme. Ber. Dt. Chem. Ges., 27:2985–2993.
Koshland DE (1958). Application of a theory of enzyme specificity to protein synthesis. PNAS., 44 (2):98–104.
Huang KF, Liu YL, Cheng WJ, Ko TP, Wang AH (2005). Crystal structures of human glutaminyl cyclase, an enzyme responsible for protein N-terminal pyroglutamate formation. PNAS., 102(37):13117-13122.
Holmes CF, Maynes JT, Perreault KR, Dawson JF, James MN (2002). Molecular enzymology underlying regulation of protein phosphatase-1 by natural toxins. Curr Med Chem., 9(22):1981-1989.
| DOI | 名称 | |
|---|---|---|
| 10.1124/mol.106.027946 | Short polybasic peptide sequences are potent inhibitors of PC5/6 and PC7: Use of positional scanning-synthetic peptide combinatorial libraries as a tool for the optimization of inhibitory sequences | 下载 |
| 10.1021/jp1054342 | Effect of association with sulfate on the electrophoretic mobility of polyarginine and polylysine | 下载 |
| 10.1002/jps.22697 | Moisture content impacts the stability of DNA adsorbed onto gold microparticles | 下载 |





