刘犇教授课题组在JOURNAL OF PHYSICAL CHEMISTRY LETTERS发表研究论文.
Promoting Effect of Heterostructured NiO/Ni on Pt Nanocatalysts toward Catalytic Hydrolysis of Ammonia Borane
Ren, XY (Ren, Xueying)[ 1 ] ; Lv, H (Lv, Hao)[ 1 ] ; Yang, S (Yang, Su)[ 1 ] ; Wang, YY (Wang, Yingying)[ 1 ] ; Li, JL (Li, Jinlong)[ 1 ] ; Wei, R (Wei, Ren)[ 1 ] ; Xu, DD (Xu, Dongdong)[ 1 ] ; Liu, B (Liu, Ben)[ 1 ]*(刘犇)
[ 1 ] Nanjing Normal Univ, Jiangsu Key Lab New Power Batteries, Jiangsu Collaborat Innovat Ctr Biomed Funct Mat, Sch Chem & Mat Sci, Nanjing 210023, Jiangsu, Peoples R China
JOURNAL OF PHYSICAL CHEMISTRY LETTERS,201912,10(23),7374-7382
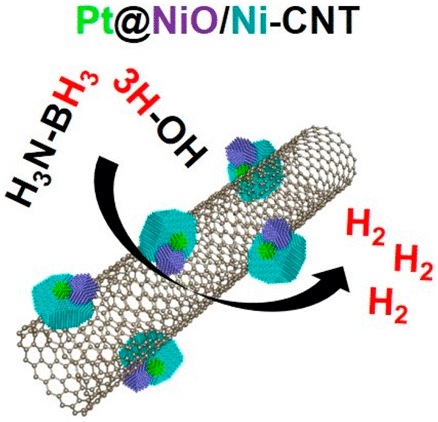
We report that heterostructured NiO/Ni nanoparticles remarkably promote the catalytic H-2 production of platinum (Pt) nanoclusters toward the hydrolysis of ammonia borane (AB). A hybrid nanocatalyst composed ultrasmall Pt nanoclusters, heterostructured NiO/Ni nanoparticles, and a carbon nanotube support (defined as Pt@NiO/Ni-CNT) is fabricated. The resultant Pt@NiO/Ni-CNT is highly efficient for room-temperature H-2 production toward catalytic hydrolysis of AB, better than the Pt@NiO-CNT and Pt@Ni-CNT with NiO or Ni alone, and the Pt@NiO/Ni without CNT support. Optimal Pt@NiO/Ni-CNT catalyst exhibits a good catalytic activity with a high TOF of 2665 mol H-2 mol(p)(t)(-1) min(-1) under ambient conditions, overtaking the activities of previously reported catalysts for AB hydrolysis. Catalytic kinetic studies indicate that compositional and structural features of the Pt@NiO/Ni-CNT synergistically accelerate the oxidative clearage of the H-OH bond from attacked H2O (the rate-determining step), thus boosting catalytic hydrolysis of AB kinetically.
文章链接:
https://pubs.acs.org/doi/10.1021/acs.jpclett.9b03080
版权与免责声明:本网页的内容由收集互联网上公开发布的信息整理获得。目的在于传递信息及分享,并不意味着赞同其观点或证实其真实性,也不构成其他建议。仅提供交流平台,不为其版权负责。如涉及侵权,请联系我们及时修改或删除。邮箱:sales@allpeptide.com