刘犇教授课题组在ANGEWANDTE CHEMIE-INTERNATIONAL EDITION发表研究论文
A Polymer Solution To Prevent Nanoclustering and Improve the Selectivity of Metal Nanoparticles for Electrocatalytic CO2 Reduction
Zhang, L (Zhang, Lei)[ 1,2 ] ; Wei, ZC (Wei, Zichao)[ 2 ] ; Thanneeru, S (Thanneeru, Srinivas)[ 2 ] ; Meng, M (Meng, Michael)[ 2 ] ; Kruzyk, M (Kruzyk, Megan)[ 2 ] ; Ung, G (Ung, Gael)[ 2 ] ; Liu, B (Liu, Ben)[ 1 ]*(刘犇) ; He, J (He, Jie)[ 2,3 ]*
[ 1 ] Nanjing Normal Univ, Collaborat Innovat Ctr Biomed Funct Mat, Sch Chem & Mat Sci, Jiangsu Key Lab New Power Batteries, Nanjing 210023, Jiangsu, Peoples R China
[ 2 ] Univ Connecticut, Dept Chem, Storrs, CT 06269 USA
[ 3 ] Univ Connecticut, Inst Mat Sci, Polymer Program, Storrs, CT 06269 USA
ANGEWANDTE CHEMIE-INTERNATIONAL EDITION,201909, DOI: 10.1002/anie.201909069
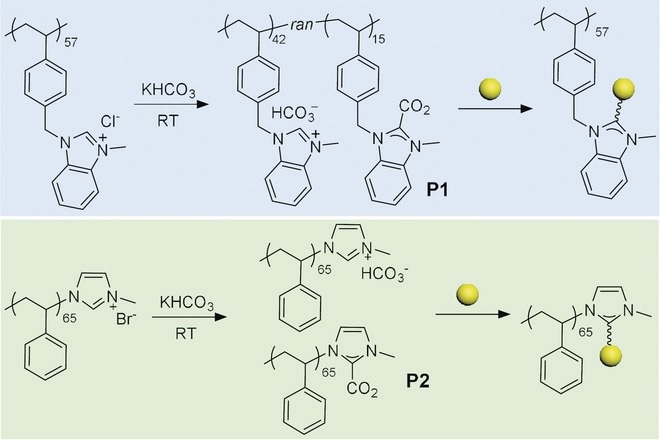
The stability of metal nanocatalysts for electrocatalytic CO2 reduction is of key importance for practical application. We report the use of two polymeric N-heterocyclic carbenes (NHC) (polydentate and monodentate) to stabilize metal nanocatalysts (Au and Pd) for efficient CO2 electroreduction. Compared with other conventional ligands including thiols and amines, metal-carbene bonds that are stable under reductive potentials prevent the nanoclustering of nanoparticles. Au nanocatalysts modified by polymeric NHC ligands show an activity retention of 86 % after CO2 reduction at -0.9 V for 11 h, while it is less than 10 % for unmodified Au. We demonstrate that the hydrophobicity of polymer ligands and the enriched surface electron density of metal NPs through sigma-donation of NHCs substantially improve the selectivity for CO2 reduction over proton.
文章链接:
https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201909069
版权与免责声明:本网页的内容由收集互联网上公开发布的信息整理获得。目的在于传递信息及分享,并不意味着赞同其观点或证实其真实性,也不构成其他建议。仅提供交流平台,不为其版权负责。如涉及侵权,请联系我们及时修改或删除。邮箱:sales@allpeptide.com