孙培培教授课题组在JOURNAL OF ORGANIC CHEMISTRY发表研究论文
Photoredox-Catalyzed Radical Cascade Reaction To Synthesize Fluorinated Pyrrolo[1,2-d]benzodiazepine Derivatives
Lian, GF (Lian, Guifang)[ 1 ] ; Li, JY (Li, Jingyu)[ 1 ] ; Liu, P (Liu, Ping)[ 1 ] ; Sun, PP (Sun, Peipei)[ 1 ]*(孙培培)
[ 1 ] Nanjing Normal Univ, Sch Chem & Mat Sci, Jiangsu Collaborat Innovat Ctr Biomed Funct Mat, Jiangsu Prov Key Lab Mat Cycle Proc & Pollut Cont, Nanjing 210023, Jiangsu, Peoples R China
JOURNAL OF ORGANIC CHEMISTRY,201907,84(14),9322-9329
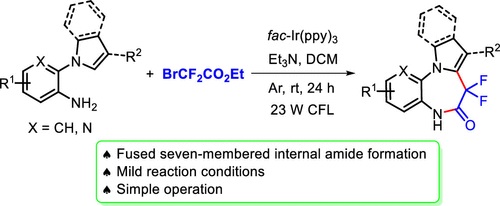
A new photoredox-catalyzed cascade reaction is described to access fluorinated pyrrolo[1,2-d]-benzodiazepine derivatives under mild conditions. In this process, single electron transfer (SET) between the excited state photocatalyst fac-Ir(ppy)(3) and ethyl bromodifluoroacetate initiated the regioselective radical addition to a wide range of 2-(1H-pyrrol-l-yl) anilines or indol-substituted anilines, followed by another SET process and intramolecular amidation.
文章链接:
https://pubs.acs.org/doi/10.1021/acs.joc.9b00937
版权与免责声明:本网页的内容由收集互联网上公开发布的信息整理获得。目的在于传递信息及分享,并不意味着赞同其观点或证实其真实性,也不构成其他建议。仅提供交流平台,不为其版权负责。如涉及侵权,请联系我们及时修改或删除。邮箱:sales@allpeptide.com