韩维教授课题组在GREEN CHEMISTRY发表研究论文
Transition-metal-free carbonylation of aryl halides with arylboronic acids by utilizing stoichiometric CHCl3 as the carbon monoxide-precursor
Xu, FN (Xu, Fangning)[ 1 ] ; Li, D (Li, Dan)[ 2 ] ; Han, W (Han, Wei)[ 1,2 ] *(韩维)
[ 1 ] Nanjing Normal Univ, Key Lab Appl Photochem, Jiangsu Collaborat Innovat Ctr Biomed Funct Mat, Jiangsu Key Lab Biofunct Mat,Sch Chem & Mat Sci, Nanjing 210023, Jiangsu, Peoples R China
[ 2 ] Changsha Univ Sci & Technol, Sch Chem & Biol Engn, Hunan Prov Key Lab Mat Protect Elect Power & Tran, Changsha 410114, Hunan, Peoples R China
GREEN CHEMISTRY,201906,21(11),2911-29152019-07-01
Under transition-metal-free conditions, carbonylative Suzuki couplings of aryl halides with arylboronic acid using stoichiometric CHCl3 as the carbonyl source has been developed. The simple, efficient, and environmentally benign method was successfully applied to the synthesis of Fenofibric acid, naphthyl phenstatin, and carbon-13 labeled biaryl ketone.
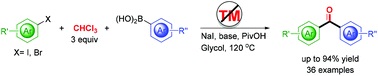
文章链接:
https://pubs.rsc.org/en/content/articlelanding/2019/GC/C9GC00598F#!divAbstract
版权与免责声明:本网页的内容由收集互联网上公开发布的信息整理获得。目的在于传递信息及分享,并不意味着赞同其观点或证实其真实性,也不构成其他建议。仅提供交流平台,不为其版权负责。如涉及侵权,请联系我们及时修改或删除。邮箱:sales@allpeptide.com