王冠博教授在ANALYTICAL CHEMISTRY发表研究论文
Wang, GB (Wang, Guanbo)[ 1 ]*(王冠博) ; Chaihu, LX (Chaihu, Lingxiao)[ 1,2 ] ; Tian, M (Tian, Meng)[ 3 ] ; Shao, XY (Shao, Xinyang)[ 4 ] ; Dai, RR (Dai, Rongrong)[ 1 ] ; de Jong, RN (de Jong, Rob N.)[ 5 ] ; Ugurlar, D (Ugurlar, Deniz)[ 6 ] ; Gros, P (Gros, Piet)[ 6 ] ; Heck, AJR (Heck, Albert J. R.)[ 7,8 ]
[ 1 ] Nanjing Normal Univ, Sch Chem & Mat Sci, Nanjing 210023, Peoples R China
[ 2 ] Shenzhen Bay Lab, Inst Cell Anal, Shenzhen 518132, Peoples R China
[ 3 ] Tsinghua Univ, Beijing Adv Innovat Ctr Struct Biol, Tsinghua Peking Joint Ctr Life Sci, Sch Life Sci, Beijing 100084, Peoples R China
[ 4 ] Peking Univ, Peking Tsinghua Ctr Life Sci, Beijing 100871, Peoples R China
[ 5 ] Genmab, NL-3584 CM Utrecht, Netherlands
[ 6 ] Univ Utrecht, Fac Sci, Bijvoet Ctr Biomol Res, Crystal & Struct Chem,Dept Chem, NL-3584 CH Utrecht, Netherlands
[ 7 ] Univ Utrecht, Bijvoet Ctr Biomol Res, Biomol Mass Spectrometry & Prote, NL-3584 CH Utrecht, Netherlands
[ 8 ] Univ Utrecht, Utrecht Inst Pharmaceut Sci, NL-3584 CH Utrecht, Netherlands
ANALYTICAL CHEMISTRY 2020, 92, 24, 15799–15805
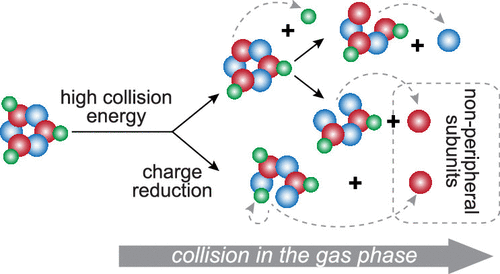
The quaternary structure is an important feature regulating protein function. Native mass spectrometry contributes to untangling quaternary structures by preserving the integrity of protein complexes in the gas phase. Tandem mass spectrometry by collision-induced dissociation (CID) can then be used to release subunits from these intact complexes, thereby providing structural information on the stoichiometry and topology. Cumulatively, such studies have revealed the preferred release of peripheral subunits during CID. In contrast, here we describe and focus on dissociation pathways that release nonperipheral subunits from hetero-complexes in CID at high collision energies. We find that nonperipheral subunits are ejected with a high propensity, as a consequence of sequential dissociation events, upon initial removal of peripheral subunits. Alternatively, nonperipheral subunits can be released directly from a charge-reduced or an elongated intact complex. As demonstrated here for a range of protein assemblies, releasing nonperipheral subunits under controlled conditions may provide unique structural information on the stoichiometry and topology of protein complexes.
文章链接:
https://pubs.acs.org/doi/10.1021/acs.analchem.0c02845
版权与免责声明:本网页的内容由收集互联网上公开发布的信息整理获得。目的在于传递信息及分享,并不意味着赞同其观点或证实其真实性,也不构成其他建议。仅提供交流平台,不为其版权负责。如涉及侵权,请联系我们及时修改或删除。邮箱:sales@allpeptide.com