400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研
源自人巨细胞病毒(HCMV)基质蛋白pp65的免疫抗原决定簇。
编号:160282
CAS号:153045-21-7
单字母:H2N-NLVPMVATV-OH
| 参考文献(References): | J.W.Gratama et al., Blood, 98, 1358 (2001) S.Szmania et al., Blood, 98, 505 (2001) K.S.Lang et al., Am. J. Physiol. Cell Physiol., 284, C200 (2003) H.Hebart et al., Exp. Hematol., 31, 966 (2003) P.S.Rohrlich et al., Hum. Immunol., 65, 514 (2004) |
CEF20 是来自巨细胞病毒 pp65(495-503)的 HLA-A*0201 限制性表位。
CEF20 is an HLA-A*0201-restricted epitope from cytomegalovirus pp65 (495-503).
该肽是来源于人巨细胞病毒(HCMV)基质蛋白pp65的免疫显性表位。
This peptide is the immunodominant epitope derived from the human cytomegalovirus (HCMV) matrix protein pp65.
Definition
The commonly used cytomegalovirus (CMV) peptides used for therapeutic approaches are pp65 and pp71. pp65 is also known as glycoprotein 64 or UL83 is a virion tegument protein and the main component of the enveloped subviral particle of CMV. The human cytomegalovirus (HCMV) tegument protein pp71 is an upper matrix protein involved in gene regulation and is encoded by gene UL82.
Discovery
The identification of DNA sequences coding for a virion phosphoprotein of 71 kDa and a viral 65-kDa polypeptide was done by Nowak B et.al, in early in 1980s1.
Structural Characteristics
CMV pp65 belongs to the herpesviridae pp65 family. HCMV contains a phosphorylated matrix protein of 65,000 apparent molecular weight (65K phosphoprotein; pp65) and a related phosphoprotein of 71,000 molecular weight (pp71). The 65K phosphoprotein is usually by far the most abundant structural component found in culture-grown purified virus particles. The 65K phosphoprotein is coded for by the 5'-terminal part of an abundant 4-kilobase (kb) mRNA. The structural protein pp65 forms about 95% of the protein mass in dense bodies. The nucleotide sequence of the entire coding domain for pp65 and pp7l,identifies separate translational reading frames and transcripts for each protein, and analyzes their RNA structures. CMV pp65 contains elements of the prototypic nuclear localization signal (NLS) in which arginine and lysine predominate within a bipartite motif in which short regions of basic amino acids are separated by 10 or more nonconserved amino acids. The nuclear localization signals of CMV pp65 consist of at least two such motifs located in the carboxy-terminal region of the polypeptide.A second NLS of CMV pp65 consists of a basic region of amino acids between aa 537 and 561; this region was termed the C-D motif by Schmolke et al,2, 3. The primary sequence of HCMV is Asn-Leu-Val-Pro-Met-Val-Ala-Thr-Val.
Mode of Action
CMV pp65 as a nucleotropic protein which enters the nucleus immediately after infection. It binds to polo-like kinase 1 (PLK-1), an enzyme important in mitosis and it is likely that the protein has specific effects on cell cycle events 4. CMV pp65 has been shown to have protein kinase activity. CMV pp65 is an immunodominant target of CD4+ and CD8+ Tcell response in CMV. CMV pp65 specific T cell predominantly produces cytokines like IFN-γ, IL-2, and TNF-α. HCMV pp71 is delivered directly to cells by infecting HCMV virions. At the start of lytic infections, it travels to the nucleus and stimulates viral IE gene expression by displacing the chromatin remodeling protein ATRX from Daxx and by mediating Daxx degradation through a rare ubiquitin-independent, proteasome-dependent process5.
Functions
CMV pp65 has been the prototypic antigen for the demonstration of CMV-specific T-cell immunity, it is likely that other proteins of CMV will be necessary for the development of a vaccine that generates humoral and cellular protection. CMV pp65-specific T-cell responses have been used for the development of other immunotherapeutic approaches to the control of CMV infection.
HCMV protein pp65 is an efficient protein carrier system into human dendritic cells 6. It is also major target for the cellular immune response. HCMV Protein pp71 disrupts major histocompatibility complex class I cell surface expression. HCMV tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. Human CMV pp65 virion protein inhibits antiviral gene expression in infected cells. HCMV pp65 mediates accumulation of HLA-DR in lysosomes and destruction of the HLA-DR α-chain 7.
References
1. Nowak B, Gmeiner A, Sarnow P, Levine AJ, Fleckenstein B (1984). Physical mapping of human cytomegalovirus genes: identification of DNA sequences coding for a virion phosphoprotein of 71 kDa and a viral 65-kDa polypeptide. Virology, 134(1):91-102.
2. Rüger B, Klages S, Walla B, Albrecht J, Fleckenstein B, Tomlinson P, Barrell B (1987). Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J Virol., 61(2):446-453.
3. Schmolke S, Drescher P, Jahn G, Plachter B (1995). Nuclear targeting of the tegument protein pp65 (UL83) of human cytomegalovirus: an unusual bipartite nuclear localization signal functions with other portions of the protein to mediate its efficient nuclear transport. J Virol., 69(2):1071-1078.
4. Zaia JA, Li X, Franck AE, Wu X, Thao L, Gallez-Hawkins G (2009). Biologic and immunologic effects of knockout of human cytomegalovirus pp65 nuclear localization signal. Clin Vaccine Immunol., 16(6):935-943.
5. Hwang J, Kalejta RF (2009). Human cytomegalovirus protein pp71 induces Daxx SUMOylation. J Virol., 83(13):6591-6598.
6. Scheller N, Furtwängler R, Sester U, Maier R, Breinig T, Meyerhans A (2008). Human cytomegalovirus protein pp65: an efficient protein carrier system into human dendritic cells. Gene Ther.,15(4):318-325.
7. Browne EP, Shenk T (2003). Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. PNAS., 100(20):11439-11444.
Definition
The CEF control peptides are 8-12 amino acids in length, with sequences derived from the human Cytomegalovirus, Epstein-Barr Virus and Influenza Virus1 These peptides are used in the stimulation of IFNg release from CD8+ T cells in individuals with defined HLA types1. They are useful as positive control peptides in several cytokine assays such as Elispot.
Discovery
CEF peptides were first selected in 2002 based on their ability to recognize CD8+ T cells1.
Classification
They are derived from epitopes of viruses and hence have antigenic properties1.
Structural Characteristics
CEF peptides are 8-11 amino acids long with sequences: GILGFVFTL (Influenza A, HLA-A2), FMYSDFHFI (Influenza A, HLA-A2), CLGGLLTMV (EBV, HLA-A2), GLCTLVAML (EBV, HLA-A2), NLVPMVATV (HCMV, HLA-A2).
Mode of action
CEF peptides are effective epitopes for CD8+ T cells2. They bind to these cells and trigger the production of IFNg.
Functions
CEF control peptides are used as positive control in Elispot assay that is used to investigate specific immune responses in various diseases including infections, cancer, allergies and autoimmune diseases2. In this case the CEF peptides ensure that the cells under study are active and viable2. Elispot is also useful in the development of vaccines especially for HIV where CEF peptides are used also as controls2.
References
1. Currier JR, Kuta EG, Turk E, Earhart LB, Loomis-Price L, Janetzki S, Ferrari G, Birx DL, Cox JH (2002). A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays, J Immunol Methods, 260, 157-172.
2. Gazagne A, Claret E, Wijdenes J, Yssel H, Bousquet F, Levy E, Vielh P, Scotte F, Goupil T, Fridman WH, Tartour E (2003). A Fluorospot assay to detect single T lymphocytes simultaneously producing multiple cytokines, J Immunol Methods, 283(1-2), 91-98.
Renae K Barr, et al. Identification of the critical features of a small peptide inhibitor of JNK activity. J Biol Chem. 2002 Mar 29;277(13):10987-97. : https://pubmed.ncbi.nlm.nih.gov/11790767
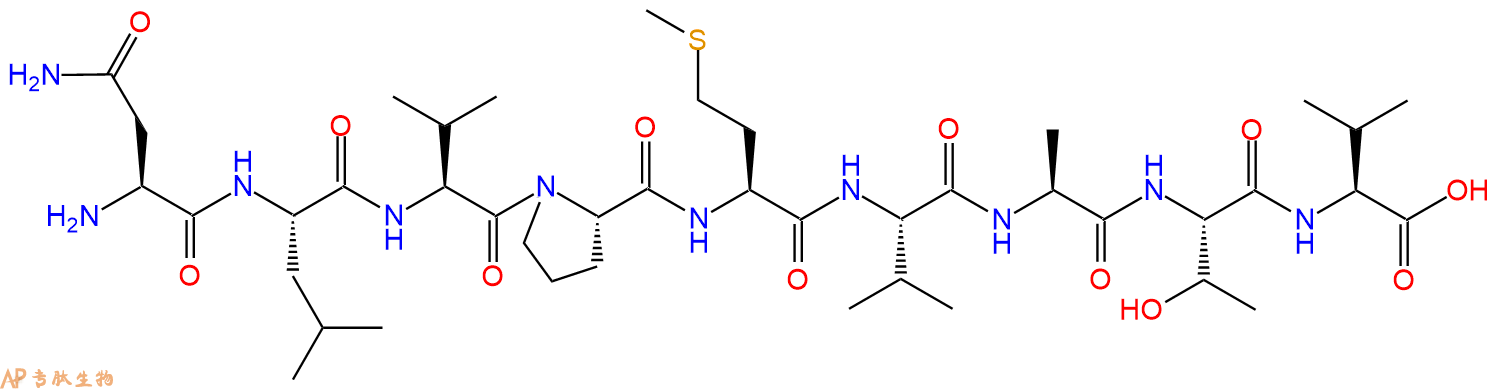
多肽H2N-Asn-Leu-Val-Pro-Met-Val-Ala-Thr-Val-COOH的合成步骤:
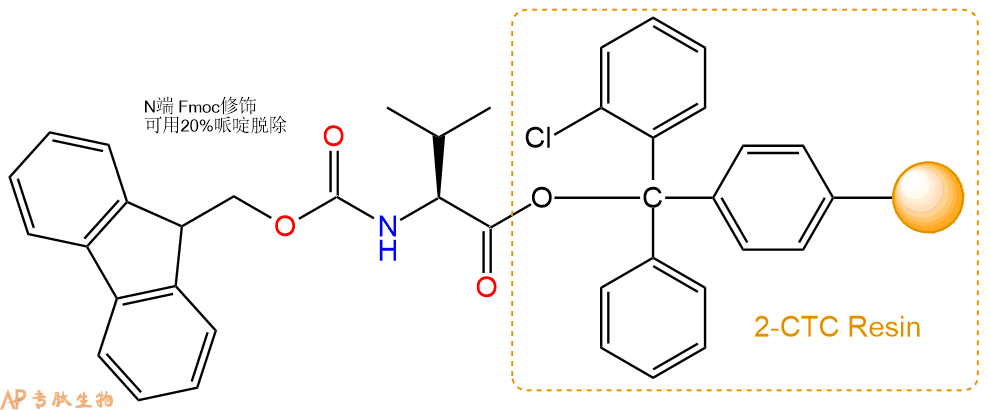
1、合成CTC树脂:称取1.11g CTC Resin(如初始取代度约为0.93mmol/g)和1.24mmol Fmoc-Val-OH于反应器中,加入适量DCM溶解氨基酸(需要注意,此时CTC树脂体积会增大好几倍,避免DCM溶液过少),再加入3.1mmol DIPEA(Mw:129.1,d:0.740g/ml),反应2-3小时后,可不抽滤溶液,直接加入1ml的HPLC级甲醇,封端半小时。依次用DMF洗涤2次,甲醇洗涤1次,DCM洗涤一次,甲醇洗涤一次,DCM洗涤一次,DMF洗涤2次(这里使用甲醇和DCM交替洗涤,是为了更好地去除其他溶质,有利于后续反应)。得到 Fmoc-Val-CTC Resin。结构图如下:
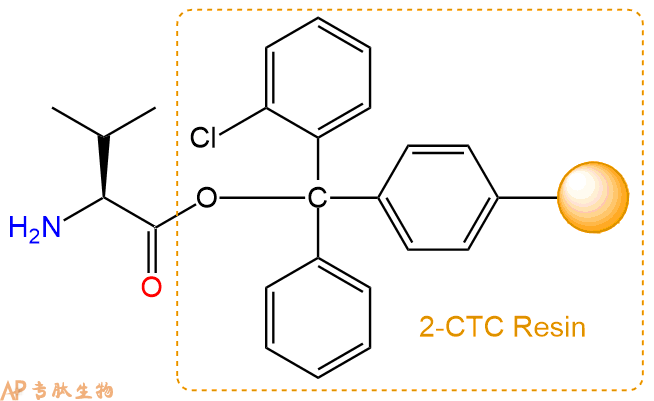
2、脱Fmoc:加3倍树脂体积的20%Pip/DMF溶液,鼓氮气30分钟,然后2倍树脂体积的DMF 洗涤5次。得到 H2N-Val-CTC Resin 。(此步骤脱除Fmoc基团,茚三酮检测为蓝色,Pip为哌啶)。结构图如下:
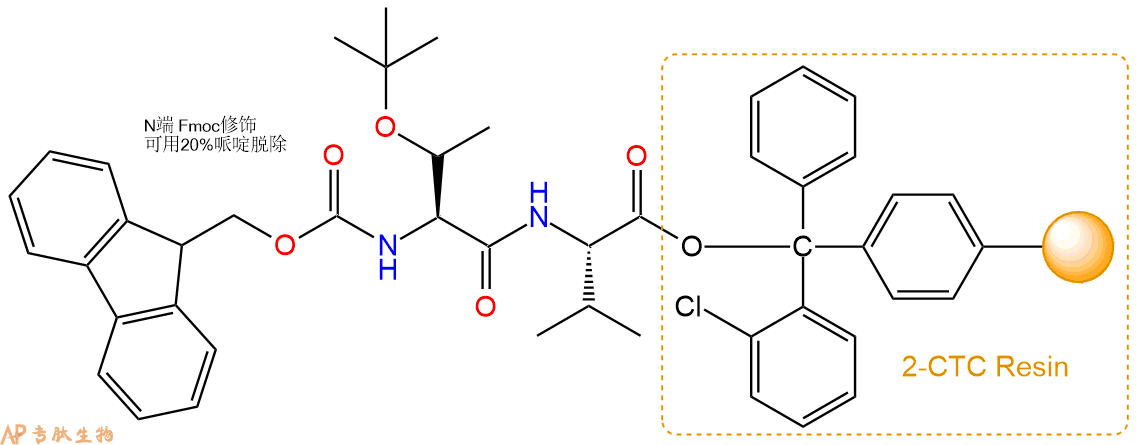
3、缩合:取3.1mmol Fmoc-Thr(tBu)-OH 氨基酸,加入到上述树脂里,加适当DMF溶解氨基酸,再依次加入6.19mmol DIPEA,2.94mmol HBTU。反应30分钟后,取小样洗涤,茚三酮检测为无色。用2倍树脂体积的DMF 洗涤3次树脂。(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。得到Fmoc-Thr(tBu)-Val-CTC Resin。氨基酸:DIPEA:HBTU:树脂=3:6:2.85:1(摩尔比)。结构图如下:
4、依次循环步骤二、步骤三,依次得到
H2N-Thr(tBu)-Val-CTC Resin
Fmoc-Ala-Thr(tBu)-Val-CTC Resin
H2N-Ala-Thr(tBu)-Val-CTC Resin
Fmoc-Val-Ala-Thr(tBu)-Val-CTC Resin
H2N-Val-Ala-Thr(tBu)-Val-CTC Resin
Fmoc-Met-Val-Ala-Thr(tBu)-Val-CTC Resin
H2N-Met-Val-Ala-Thr(tBu)-Val-CTC Resin
Fmoc-Pro-Met-Val-Ala-Thr(tBu)-Val-CTC Resin
H2N-Pro-Met-Val-Ala-Thr(tBu)-Val-CTC Resin
Fmoc-Val-Pro-Met-Val-Ala-Thr(tBu)-Val-CTC Resin
H2N-Val-Pro-Met-Val-Ala-Thr(tBu)-Val-CTC Resin
Fmoc-Leu-Val-Pro-Met-Val-Ala-Thr(tBu)-Val-CTC Resin
H2N-Leu-Val-Pro-Met-Val-Ala-Thr(tBu)-Val-CTC Resin
Fmoc-Asn(Trt)-Leu-Val-Pro-Met-Val-Ala-Thr(tBu)-Val-CTC Resin
以上中间结构,均可在专肽生物多肽计算器-多肽结构计算器中,一键画出。
最后再经过步骤二得到 H2N-Asn(Trt)-Leu-Val-Pro-Met-Val-Ala-Thr(tBu)-Val-CTC Resin,结构如下:
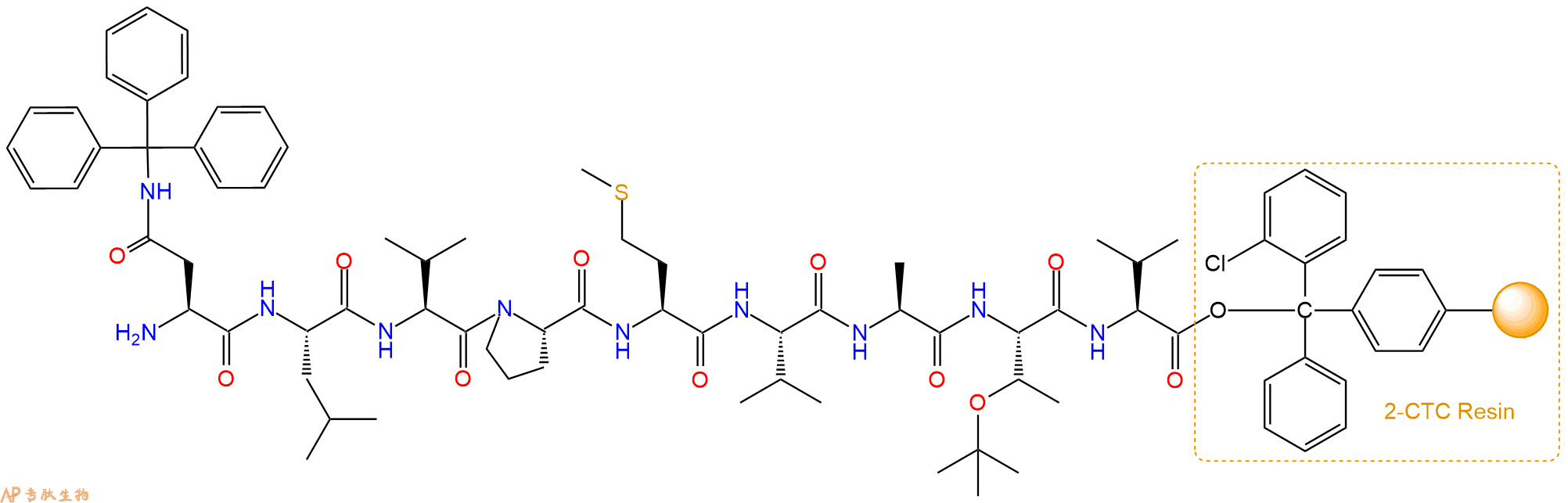
5、切割:6倍树脂体积的切割液(或每1g树脂加8ml左右的切割液),摇床摇晃 2小时,过滤掉树脂,用冰无水乙醚沉淀滤液,并用冰无水乙醚洗涤沉淀物3次,最后将沉淀物放真空干燥釜中,常温干燥24小试,得到粗品H2N-Asn-Leu-Val-Pro-Met-Val-Ala-Thr-Val-COOH。结构图见产品结构图。
切割液选择:1)TFA:H2O=95%:5%、TFA:H2O=97.5%:2.5%
2)TFA:H2O:TIS=95%:2.5%:2.5%
3)三氟乙酸:茴香硫醚:1,2-乙二硫醇:苯酚:水=87.5%:5%:2.5%:2.5%:2.5%
(前两种适合没有容易氧化的氨基酸,例如Trp、Cys、Met。第三种适合几乎所有的序列。)
6、纯化冻干:使用液相色谱纯化,收集目标峰液体,进行冻干,获得蓬松的粉末状固体多肽。不过这时要取小样复测下纯度 是否目标纯度。
7、最后总结:
杭州专肽生物技术有限公司(ALLPEPTIDE https://www.allpeptide.com)主营定制多肽合成业务,提供各类长肽,短肽,环肽,提供各类修饰肽,如:荧光标记修饰(CY3、CY5、CY5.5、CY7、FAM、FITC、Rhodamine B、TAMRA等),功能基团修饰肽(叠氮、炔基、DBCO、DOTA、NOTA等),同位素标记肽(N15、C13),订书肽(Stapled Peptide),脂肪酸修饰肽(Pal、Myr、Ste),磷酸化修饰肽(P-Ser、P-Thr、P-Tyr),环肽(酰胺键环肽、一对或者多对二硫键环),生物素标记肽,PEG修饰肽,甲基化修饰肽
以上所有内容,为专肽生物原创内容,请勿发布到其他网站上。





