400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研
血小板反应蛋白(TSP-1)衍生的CD36结合基序是一种生物活性肽。
编号:425126
CAS号:138849-26-0
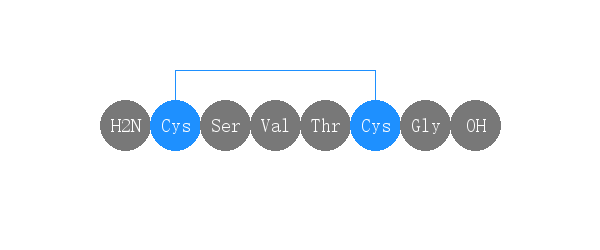
单字母:H2N-CSVTCG-OH(Disulfide Bridge:C1-C5)
| 编号: | 425126 |
| 中文名称: | 血小板反应蛋白(TSP-1)衍生的CD36结合基序:Thrombospondin - derived Peptide |
| 英文名: | Thrombospondin - derived Peptide |
| CAS号: | 138849-26-0 |
| 单字母: | H2N-CSVTCG-OH(Disulfide Bridge:C1-C5) |
| 三字母: | H2N-Cys-Ser-Val-Thr-Cys-Gly-OH(Disulfide Bridge:Cys1-Cys5) |
| 氨基酸个数: | 6 |
| 分子式: | C20H34N6O9S2 |
| 平均分子量: | 566.65 |
| 精确分子量: | 566.18 |
| 等电点(PI): | - |
| pH=7.0时的净电荷数: | 2.91 |
| 平均亲水性: | -0.72 |
| 疏水性值: | 1.22 |
| 消光系数: | - |
| 标签: | 肿瘤(Cancer) 二硫键环肽 靶向多肽 |
血小板反应蛋白(TSP-1)衍生的CD36结合基序是一种生物活性肽。(该肽来源于血小板反应蛋白,代表负责血小板反应蛋白-CD36相互作用的结合基序。它通过二硫键环化。血小板反应蛋白是一种基质结合糖蛋白,参与癌症转移、肿瘤粘附和血管生成。该肽已被证明竞争性抑制血小板聚集和肿瘤转移。)
Peptide H-CSVTCG-OH is a Research Peptide with significant interest within the field academic and medical research. Recent citations using H-CSVTCG-OH include the following: Human microvascular endothelial cells adhere to thrombospondin-1 via an RGD/CSVTCG domain independent mechanism ZS Chen, J Pohl , TJ Lawley, RA Swerlick - Journal of investigative , 1996 - Elsevierhttps://www.sciencedirect.com/science/article/pii/S0022202X15424303 Identification of SVTCG in thrombospondin as the conformation-dependent, high affinity binding site for its receptor, CD36 WX Li, RJ Howard , LL Leung - Journal of Biological Chemistry, 1993 - ASBMBhttps://www.jbc.org/article/S0021-9258(19)85403-0/abstract Structure and Function of Thrombospondins W Frazier - Trends in Glycoscience and Glycotechnology, 1992 - jstage.jst.go.jphttps://www.jstage.jst.go.jp/article/tigg1989/4/16/4_16_152/_article/-char/ja/ The localization of thrombospondin-1 (TSP-1), cysteine-serine-valine-threonine-cysteine-glycine (CSVTCG) TSP receptor, and matrix metalloproteinase-9 T Wakiyama, T Shinohara, T Shirakusa - Histology and , 2001 - hh.um.eshttps://www.hh.um.es/pdf/Vol_16/16_2/The%20localization%20of%20thrombospondin-1.pdf a Motif Shared by the Malaria Circumsporozoite Protein and Thrombospondin is Mediated Exclusively by its Glycosaminoglycan-Binding Region and Not by CSVTCG SM Gantt , P Clavijo, X Baic, J Esko - may be from any type of , 2000 - search.proquest.comhttps://search.proquest.com/openview/62ac16a7ea361a737bcf29c2fb6e2573/1.pdf?pq-origsite=gscholar&cbl=18750&diss=y#page=58 adhesion to a motif shared by the malaria circumsporozoite protein and thrombospondin is mediated by its glycosaminoglycan-binding region and not by CSVTCG SM Gantt , P Clavijo, X Bai, JD Esko, P Sinnis - Journal of Biological , 1997 - ASBMBhttps://www.jbc.org/article/S0021-9258(18)38944-0/abstract Plasmodium falciparum-infected erythrocyte adhesion to the type 3 repeat domain of thrombospondin-1 is mediated by a modified band 3 protein S Eda, J Lawler , IW Sherman - Molecular and biochemical parasitology, 1999 - Elsevierhttps://www.sciencedirect.com/science/article/pii/S0166685199000584 A conserved peptide sequence of the Plasmodium falciparum circumsporozoite protein and antipeptide antibodies inhibit Plasmodium berghei sporozoite invasion of S Chatterjee, M Wery, P Sharma - Infection and , 1995 - Am Soc Microbiolhttps://journals.asm.org/doi/abs/10.1128/iai.63.11.4375-4381.1995 Histidine-rich glycoprotein inhibits the antiangiogenic effect of thrombospondin-1 R Simantov , M Febbraio , R Crombie - The Journal of , 2001 - Am Soc Clin Investighttps://www.jci.org/articles/view/9061 Identification of cell adhesive active sites in the N-terminal domain of thrombospondin-1 P CLEZARDIN , J LAWLER , J AMIRAL - Biochemical , 1997 - portlandpress.comhttps://portlandpress.com/biochemj/article-abstract/321/3/819/34171 Heparin-and sulfatide-binding peptides from the type I repeats of human thrombospondin promote melanoma cell adhesion. N Guo, HC Krutzsch, E Negre, T Vogel - Proceedings of the , 1992 - National Acad Scienceshttps://www.pnas.org/doi/abs/10.1073/pnas.89.7.3040 Identification of active peptide sequences in the carboxyl-terminal cell binding domain of human thrombospondin-1. MD Kosfeld, WA Frazier - Journal of Biological Chemistry, 1992 - Elsevierhttps://www.sciencedirect.com/science/article/pii/S0021925818419904 Cell attachment activity of the carboxyl-terminal domain of human thrombospondin expressed in Escherichia coli. MD Kosfeld, TV Pavlopoulos, WA Frazier - Journal of Biological Chemistry, 1991 - Elsevierhttps://www.sciencedirect.com/science/article/pii/S0021925818542228 THE 1998 MOYER AWARD Characteristics of Thrombospondin-1 and Its Cysteine-Serine-Valine-Threonine-Cysteine-Glycine Receptor in Burn Wounds JJ Roth, WB Hughes, FA DeClement - Journal of Burn Care , 1998 - academic.oup.comhttps://academic.oup.com/jbcr/article-abstract/19/6/487/4757981 Localization of thrombospondin-1 and its cysteine-serine-valine-threonine-cysteine-glycine receptor in colonic anastomotic healing tissue JJ Roth, MA Buckmire, RH Rolandelli - Histology and , 1998 - hh.um.eshttps://www.hh.um.es/pdf/Vol_13/13_4/Localization%20of%20thrombospondin-l%20and%20its%20cysteine-serine-valine-threonine-cysteine-glycine%20recept.pdf Thrombospondin 1 and its specific cysteine-serine-valine-threonine-cysteine-glycine receptor in fetal wounds JJ Roth, JJ Sung, MS Granick , MP Solomon - Annals of plastic , 1999 - journals.lww.comhttps://journals.lww.com/annalsplasticsurgery/abstract/1999/05000/Thrombospondin_1_and_Its_Specific.16.aspx A CD36-binding peptide from thrombospondin-1 can stimulate resorption by osteoclasts in vitro JA Carron, SC Wagstaff, JA Gallagher - and biophysical research , 2000 - Elsevierhttps://www.sciencedirect.com/science/article/pii/S0006291X00925749 Cloning and characterization of angiocidin, a tumor cell binding protein for thrombospondin-1 J Zhou, VL Rothman, I Sargiannidou - Journal of cellular , 2004 - Wiley Online Libraryhttps://onlinelibrary.wiley.com/doi/abs/10.1002/jcb.20076 Cloning and characterization of CSVTCG-specific receptor of thrombospondin-1 J Zhou - 2002 - search.proquest.comhttps://search.proquest.com/openview/4936bad0f3ba407c917959e11d28a4ba/1?pq-origsite=gscholar&cbl=18750&diss=y Thrombin-stimulated calcium mobilization is inhibited by thrombospondin via CD36 J Enenstein, K Gupta, GM Vercellotti - Experimental cell research, 1998 - Elsevierhttps://www.sciencedirect.com/science/article/pii/S0014482797938635 The Functional Significance of the CSVTCG-Specific Receptor in Breast Carcinoma Progression I Sargiannidou , G Tuszynski - 2002., 2002 - apps.dtic.milhttps://apps.dtic.mil/sti/citations/tr/ADA405490 Identification and characterization of a tumor cell receptor for CSVTCG, a thrombospondin adhesive domain. GP Tuszynski, VL Rothman, M Papale - The Journal of cell , 1993 - rupress.orghttps://rupress.org/jcb/article-abstract/120/2/513/14638 Biological activities of peptides and peptide analogues derived from common sequences present in thrombospondin, properdin, and malarial proteins. GP Tuszynski, VL Rothman, AH Deutch - The Journal of cell , 1992 - rupress.orghttps://rupress.org/jcb/article-abstract/116/1/209/14270 Stimulation of platelet activation and aggregation by a carboxyl-terminal peptide from thrombospondin binding to the integrin-associated protein receptor DJ Dorahy, RF Thorne, JV Fecondo - Journal of Biological , 1997 - ASBMBhttps://www.jbc.org/article/S0021-9258(19)77610-8/abstract Angiocidin inhibitory peptides decrease tumor burden in a murine colon cancer model C Liebig, N Agarwal, GE Ayala , G Verstovsek - Journal of Surgical , 2007 - Elsevierhttps://www.sciencedirect.com/science/article/pii/S0022480407001163 77: Inhibition of angiocidin by two novel peptides decreases tumor burden in an orthotopic nude mouse model C Liebig, N Agarwal, G Ayala - Journal of , 2007 - journalofsurgicalresearch.comhttps://www.journalofsurgicalresearch.com/article/S0022-4804(06)00707-4/abstract Inhibition of Angiogenesis by the First Type I Repeat Peptides of Thrombospondin-1 BI Yoo, GB Jeong - Korean Journal of Physical Anthropology, 2015 - e-aba.orghttps://e-aba.org/DOIx.php?id=10.11637/kjpa.2015.28.4.223 Thrombospondins as anti-angiogenic therapeutic agents B Vailh, JJ Feige - Current pharmaceutical design, 2003 - ingentaconnect.comhttps://www.ingentaconnect.com/content/ben/cpd/2003/00000009/00000007/art00008 Human platelet glycoprotein IIIb binds to thrombospondin fragments bearing the C-terminal region, and/or the type I repeats (CSVTCG motif), but not to the N B Catimel, L Leung , H El Ghissasi, N Mercier - Biochemical , 1992 - portlandpress.comhttps://portlandpress.com/biochemj/article-abstract/284/1/231/37093 Thrombospondin sequence motif (CSVTCG) is responsible for CD36 binding AS Asch, S Silbiger, E Heimer, RL Nachman - Biochemical and biophysical , 1992 - Elsevierhttps://www.sciencedirect.com/science/article/pii/0006291X9291860S INTERACTIONS OF THROMBOSPONDIN WITH CELLS A PART - Thrombospondin, 1993 - books.google.comhttps://books.google.com/books?hl=en&lr=&id=Hanf7fhm_tsC&oi=fnd&pg=PA91&dq=(%22H-CSVTCG-OH%22+OR+%22CSVTCG%22+OR+%22NH2-Cys-Ser-Val-Thr-Cys-Gly-OH%22)+AND+peptide&ots=5QKzsRxT-P&sig=rdbLm8_Kg3vhMNQEczanw_NjVTI
化学预防肽是有助于预防疾病(例如癌症或糖尿病)的发作或发展的肽。这些肽可以源自天然来源,例如大豆或牛奶,也可以来自肽模拟物的设计,也可以源自使用合成肽进行的肽筛选。据认为,这些肽中的某些可以充当细胞周期的调节剂,其调节使细胞通过复制周期前进所需的蛋白质的产生和功能。另外,现在有越来越多的证据表明特定的饮食模式,食物和饮料以及其他饮食物质可以而且确实可以预防癌症。越来越多的流行病学研究表明,食物,营养和身体活动在预防和改变癌症过程中很重要。包括植物蛋白酶抑制剂,乳铁蛋白,乳铁蛋白,凝集素和lunasin在内的不同类型的食物蛋白和多肽似乎起着化学预防剂的作用。如今,蛋白质和多肽被认为是一组营养保健品,在预防癌症的不同阶段(包括起始,促进和进展)方面显示出潜力。此外,已经发现在植物中发现的一些蛋白酶抑制剂,例如豆类和大豆,是有效的癌发生抑制剂。致癌作用是引发和促进癌症的过程。 Bowman-Birk抑制剂和Kunitz胰蛋白酶抑制剂就在其中。目前,这些化合物在致癌作用中的生物学功能主要归因于抑制癌细胞的侵袭和转移,但是,其作用机理仍不完全清楚,需要进一步研究以充分阐明它们。
二硫键广泛存在与蛋白结构中,对稳定蛋白结构具有非常重要的意义,二硫键一般是通过序列中的2个Cys的巯基,经氧化形成。
形成二硫键的方法很多:空气氧化法,DMSO氧化法,过氧化氢氧化法等。
二硫键的合成过程, 可以通过Ellman检测以及HPLC检测方法对其反应进程进行监测。
如果多肽中只含有1对Cys,那二硫键的形成是简单的。多肽经固相或液相合成,然后在pH8-9的溶液中进行氧化。
当需要形成2对或2对以上的二硫键时,合成过程则相对复杂。尽管二硫键的形成通常是在合成方案的最后阶段完成,但有时引入预先形成的二硫化物是有利于连合或延长肽链的。通常采用的巯基保护基有trt, Acm, Mmt, tBu, Bzl, Mob, Tmob等多种基团。我们分别列出两种以2-Cl树脂和Rink树脂为载体合成的多肽上多对二硫键形成路线:
二硫键反应条件选择
二硫键即为蛋白质或多肽分子中两个不同位点Cys的巯基(-SH)被氧化形成的S-S共价键。 一条肽链上不同位置的氨基酸之间形成的二硫键,可以将肽链折叠成特定的空间结构。多肽分 子通常分子量较大,空间结构复杂,结构中形成二硫键时要求两个半胱氨酸在空间距离上接近。 此外,多肽结构中还原态的巯基化学性质活泼,容易发生其他的副反应,而且肽链上其他侧链 也可能会发生一系列修饰,因此,肽链进行修饰所选取的氧化剂和氧化条件是反应的关键因素, 反应机理也比较复杂,既可能是自由基反应,也可能是离子反应。
反应条件有多种选择,比如空气氧化,DMSO氧化等温和的氧化过程,也可以采用H2O2,I2, 汞盐等激烈的反应条件。
空气氧化法: 空气氧化法形成二硫键是多肽合成中最经典的方法,通常是将巯基处于还原态的多肽溶于水中,在近中性或弱碱性条件下(PH值6.5-10),反应24小时以上。为了降低分子之间二硫键形成的可能,该方法通常需要在低浓度条件下进行。
碘氧化法:将多肽溶于25%的甲醇水溶液或30%的醋酸水溶液中,逐滴滴加10-15mol/L的碘进行氧化,反应15-40min。当肽链中含有对碘比较敏感的Tyr、Trp、Met和His的残基时,氧化条件要控制的更精确,氧化完后,立即加入维生素C或硫代硫酸钠除去过量的碘。 当序列中有两对或多对二硫键需要成环时,通常有两种情况:
自然随机成环: 序列中的Cys之间随机成环,与一对二硫键成环条件相似;
定点成环: 定点成环即序列中的Cys按照设计要求形成二硫键,反应过程相对复杂。在 固相合成多肽之前,需要提前设计几对二硫键形成的顺序和方法路线,选择不同的侧链 巯基保护基,利用其性质差异,分步氧化形成两对或多对二硫键。 通常采用的巯基保护 基有trt, Acm, Mmt, tBu, Bzl, Mob, Tmob等多种基团。
靶向多肽可以根据其功能和用途分为不同的类别。在PDC(多肽偶联药物)中,靶向多肽通常被分为细胞穿透肽和细胞靶向肽两大类。
细胞穿透肽:这类多肽能够跨越细胞膜,转运具有生物活性的大分子物质,如多肽、蛋白质、核酸等化学药物,使其顺利进入细胞。一些常见的细胞穿透肽包括Pep-1、Pentratin、PepFact14、Transportan等。
细胞靶向肽:这类多肽的作用主要是引导化学药物或生物活性分子与特定类型的细胞结合,以提高其靶向性和治疗效率。常见的细胞靶向肽包括PEGA、生长激素抑制素类似物、蛙皮素类似物、RGD肽类等。





