400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研
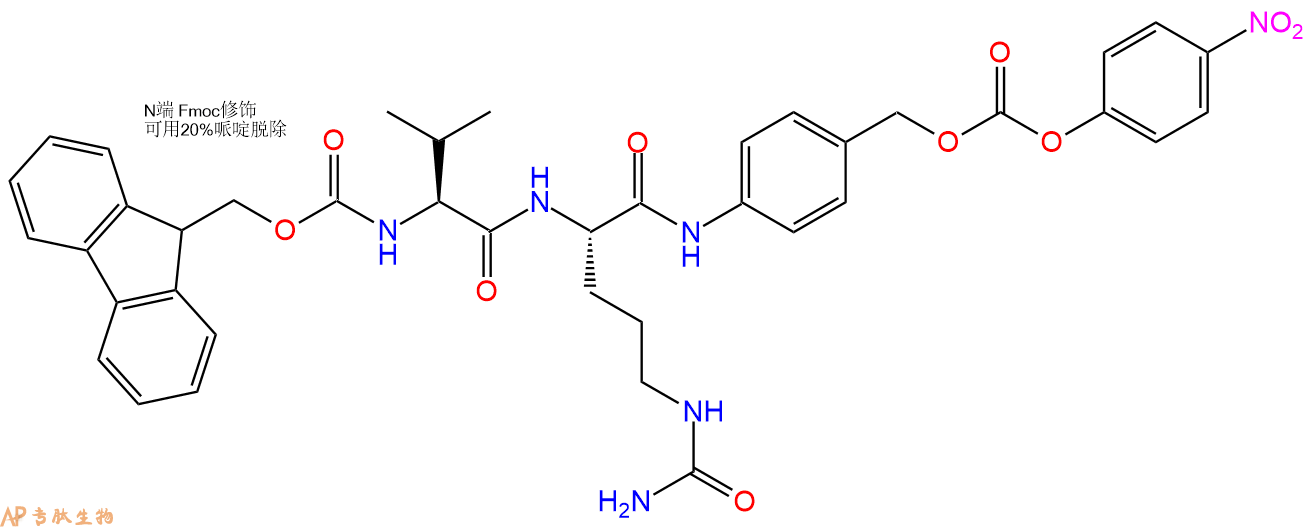
Fmoc-Val-Cit-PAB-PNP是一种可裂解的ADC接头,用于抗体-药物偶联物中。Val-Cit连接体被设计为被组织蛋白酶B切割。当与含胺有效载荷反应时,PNP基团是一个良好的离去基团。
编号:436702
CAS号:863971-53-3
单字母:Fmoc-V-Cit-PAB-PNP
| 编号: | 436702 |
| 中文名称: | Peptide Linkers(ADC Linkers):Fmoc-Val-Cit-PAB-PNP |
| 英文名: | Fmoc-Val-Cit-PAB-PNP |
| CAS号: | 863971-53-3 |
| 单字母: | Fmoc-V-Cit-PAB-PNP |
| 三字母: | Fmoc N端Fmoc保护,9-芴甲氧羰基(Fmoc)是伯胺和仲胺的保护基团。在固相肽合成(SPPS)中,它是氨基酸主链的常用保护基,因为它可以很容易地用哌啶去除,而不会干扰树脂和肽之间的酸不稳定连接体。 -ValL-缬氨酸:valine。系统命名为(2S)-氨基-3-甲基丁酸。是编码氨基酸。是哺乳动物的必需氨基酸。符号:V,Val。在某些放线菌素如缬霉素中存在 D-缬氨酸。 -Cit瓜氨酸:citrulline。系统命名为(2S)-氨基-5-脲基戊酸。首先在西瓜汁中发现。L-瓜氨酸是动物体内氨基酸代谢、尿素循环中的重要中间物。L-瓜氨酸是一种α-氨基酸,由于翻译后修饰而存在于某些蛋白质中。血循环中的瓜氨酸是肠道功能状况的生物标志物。 -PAB暂无说明 -PNPPNP |
| 氨基酸个数: | 3 |
| 分子式: | C40H42O10N6 |
| 平均分子量: | 766.8 |
| 精确分子量: | 766.3 |
| 等电点(PI): | - |
| pH=7.0时的净电荷数: | - |
| 平均亲水性: | -1.5 |
| 疏水性值: | 4.2 |
| 消光系数: | - |
| 标签: | 氨基酸衍生物肽 Peptide linkers (ADC Linkers) 三肽 |
Fmoc-Val-Cit-PAB-PNP是一种可裂解的ADC接头,用于抗体-药物偶联物中。Val-Cit连接体被设计为被组织蛋白酶B切割。当与含胺有效载荷反应时,PNP基团是一个良好的离去基团。
Fmoc-Val-Cit-PAB-PNP is a a cleavable ADC linker used in antibody drug conjugate. The Val-Cit linker was designed to be cleaved by cathepsin B. PNP group is a good leaving group when reacting with amine bearing payload.
ADC linkers的介绍
ADC linkers are one of the three main components of the antibody drug conjugates (ADC) that connect an antibody with a potent drug (payload) through a chemical bond.
Role of ADC Linkers
ADC linkers play key roles in determining the overall success of the Antibody Drug Conjugates. One of the main challenges in developing a safe and effective ADC drug (Figure 1) is the assembly of a desirable chemical linker between cytotoxic payload and mAb. A well-designed ADC linker can help the antibody to selectively deliver and accurately release the cytotoxic drug at tumor sites. It also plays critical roles in an ADCs' stability during preparation, storage, and systemic circulation. A stable ADC drug ensures that less cytotoxic payloads fall off before reaching tumor cells, increasing safety, and limiting dose.
There are two main categories of ADC linkers in current ADC drugs, cleavable linkers and non-cleavable linkers.
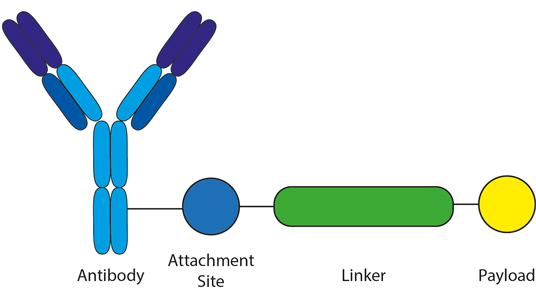
Figure 1. There are three major components of an ADC drug; the antibody used, the linker, and the payload to be delivered.
Cleavable linkers are designed to be stable in the bloodstream and then release the payload once in the cell. Cleavable linker types include enzymatically-cleavable peptide linkers, acid sensitive hydrazone linkers, and glutathione-sensitive disulfide linkers.
Example of Cleavable Linkers in ADC
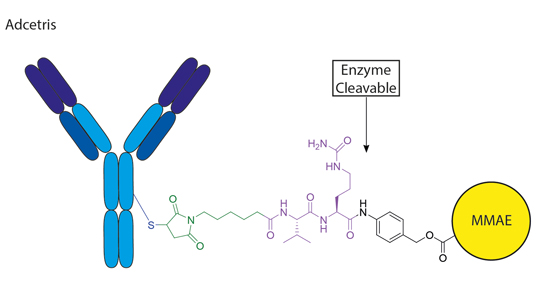
Figure 2. Adcetris with enzymatically cleavable val-cit linkage.
The non-cleavable linkers, such as SMCC, rely on lysosomal degradation within the cell to release the drug payload.
A summary of linker types is provided in Table 1.
Table 1. Linker type, mechanism and advantages of cleavable and non-cleavable linkers.
| Linker | Strategy | Mechanism | Advantages |
| Cleavable Linker | Peptides | Selectively cleaved by hydrolytic enzymes | Stability during circulation Hydrophilicity Traceless release of payload |
| Hydrazone | Acid-sensitive environments endosomal (pH = 5-6) lysosomal (pH = 4.8) | Intracellular release of payload | |
| Disulfide | Intracellular reducing molecules, such as glutathione | Intracellular release of payload | |
| Non-cleavable Linker | Stable linker without cleavage mechanism | Unknown mechanism of lysosomal cleavage | Stability during circulation |
An interesting part of the ongoing discussion about linker stability is whether the payload can or should be released into the area outside of the tumor cell. This effect, referred to as the ‘bystander effect’, is seen by some as a beneficial attribute for an ADC to display. However, recent studies indicate that, depending on the linker and payload combination, this mechanism may not be essential, and ADCs can be cleaved extracellularly or via other mechanisms.
PEG Increases the Solubility of ADC Linkers
The solubility of the linker is another parameter that has been explored using Monodispered PEG chains. Two of the latest ADCs to be approved, Trodelvy and Zynlonta, were developed with PEG moiety as part of their linker technology to improve solubility and stability in vivo.
Example of ADC Linkers with PEG Chain
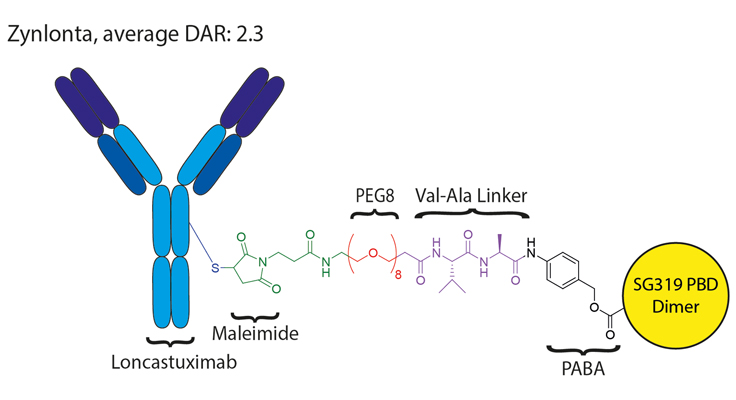
Figure 3. Zynlonta, shown above, has several unique features including a maleimide group for attachment to the mAbs, a PEG8 linker for solubility, and a cleavable Val-Ala section bound to the drug SG3199.
Journal Reference:
Halford, "A new generation of antibody-drug conjugates for cancer patients", Chemical and Engineering News, vol 98, 14, (2020) https://cen.acs.org/biological-chemistry/cancer/new-generation-antibody-drug-conjugates/98/i14
Staudacher, Brown, "Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required?", Br J Cancer 117, (2017): 1736–1742, https://www.nature.com/articles/bjc2017367#citeas
Joubert, et al., "Antibody-Drug Conjugates: The Last Decade", Pharmaceuticals (Basel, Switzerland) vol 13,9, (2020): 245-276, https://pubmed.ncbi.nlm.nih.gov/32937862/





