400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研
Arg-Gly-Asp-Ser (TFA) 是整联蛋白结合序列,抑制整联蛋白受体 (integrin receptor) 功能。Arg-Gly-Asp-Ser (TFA) 直接并特异性地结合 pro-caspase-8,pro-caspase-9 和 pro-caspase-3,但不与 pro-caspase-1 结合。
编号:182999
CAS号:91037-65-9
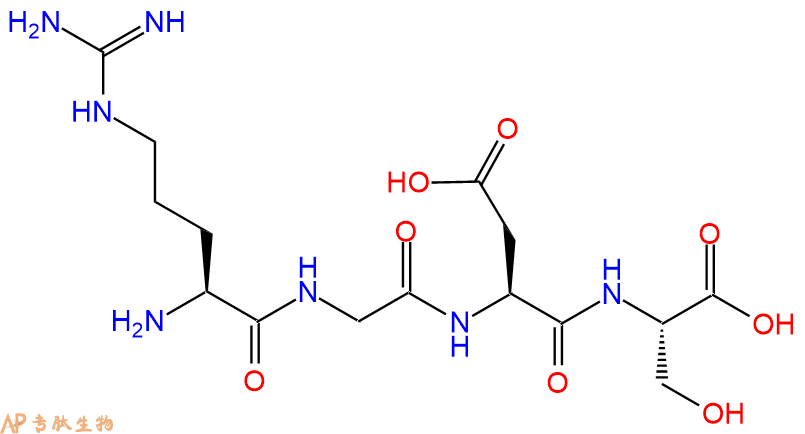
单字母:H2N-RGDS-OH
| 编号: | 182999 |
| 中文名称: | 纤维连接蛋白四肽RGDS、Fibronectin tetrapeptide、The integrin-blocking peptide |
| 英文名: | The integrin-blocking peptide |
| 英文同义词: | RGDS peptide (TFA); Fibronectin tetrapeptide (TFA) |
| CAS号: | 91037-65-9 |
| 单字母: | H2N-RGDS-OH |
| 三字母: | H2N N端氨基:N-terminal amino group。在肽或多肽链中含有游离a-氨基的氨基酸一端。在表示氨基酸序列时,通常将N端放在肽链的左边。 -ArgL-精氨酸:arginine。系统命名为(2S)-氨基-5-胍基戊酸。在生理条件下带正电荷,为编码氨基酸。是幼小哺乳动物的必需氨基酸。符号:R,Arg。 -Gly甘氨酸:glycine。系统命名为 2-氨基乙酸。是编码氨基酸中没有旋光性的最简单的氨基酸,因具有甜味而得名。符号:G,Gly。 -AspL-天冬氨酸:aspartic acid。系统命名为(2S)-氨基-丁二酸。是编码氨基酸,又是神经递质。符号:D,Asp。D-天冬氨酸存在于多种细菌的细胞壁和短杆菌肽A中。 -SerL-丝氨酸:serine。系统命名为(2S)-氨基-3-羟基丙酸。是编码氨基酸。因可从蚕丝中获得而得名。符号:S,Ser。在丝原蛋白及某些抗菌素中含有 D-丝氨酸。 -OHC端羧基:C-terminal carboxyl group。在肽或多肽链中含有游离羧基的氨基酸一端。在表示氨基酸序列时,通常将C端放在肽链的右边。 |
| 氨基酸个数: | 4 |
| 分子式: | C15H27N7O8 |
| 平均分子量: | 433.42 |
| 精确分子量: | 433.19 |
| 等电点(PI): | 10.56 |
| pH=7.0时的净电荷数: | 0.98 |
| 平均亲水性: | 2.1 |
| 疏水性值: | -2.3 |
| 外观与性状: | 白色粉末状固体 |
| 消光系数: | - |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 纯度: | 95%、98% |
| 盐体系: | 可选TFA、HAc、HCl或其它 |
| 生成周期: | 2-3周 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 肿瘤(Cancer) 整合素家族(Integrins) RGD、RAD肽 |
Arg-Gly-Asp-Ser (TFA) 是整联蛋白结合序列,抑制整联蛋白受体 (integrin receptor) 功能。Arg-Gly-Asp-Ser (TFA) 直接并特异性地结合 pro-caspase-8,pro-caspase-9 和 pro-caspase-3,但不与 pro-caspase-1 结合。
Arg-Gly-Asp-Ser (TFA) is an integrin binding sequence that inhibits integrin receptor function. Arg-Gly-Asp-Ser (TFA) directly and specifically bind pro-caspase-8, pro-caspase-9 and pro-caspase-3, while it does not bind pro-caspase-1
RGDS肽含有RGD基序,该基序是整合素的拮抗剂。
RGDS peptide contains the RGD motif which is an antagonist of integrin .
整合素是细胞表面受体,其识别存在于各种细胞外基质蛋白中的RGD基序,如纤连蛋白、玻璃体凝集素、层粘连蛋白、纤维蛋白原、von Willebrand因子、骨桥蛋白、血小板反应蛋白和胶原以及崩解蛋白中。整合素的激活触发不同的信号,调节细胞粘附、迁移、存活、凋亡以及血管生成、血栓形成和骨质疏松等过程。
Integrins are cell-surface receptors that recognize RGD motifs present in various extracellular matrix proteins such as fibronectin, vitronectin, laminin, fibrinogen, von Willebrand factor, osteopontin, thrombospondin, and collagen as well as in disintegrins. Integrins activation triggers different signals regulating cell adhesion, migration, survival, apoptosis as well as processes such as angiogenesis, thrombosis and osteoporosis .
在SK-MEL-110细胞中,500μg/ml的RGDS显著抑制人成纤维细胞生长因子-2(FGF-2)诱导的黑色素瘤细胞增殖。此外,RGDS处理(48小时,500μg/ml)使亚G1期细胞的百分比从3%(单独使用FGF-2)增加到13.2%(使用FGF-2和RGDS),表明在存在FGF-2的情况下,RGDS可能诱导黑色素瘤细胞凋亡。
In SK-MEL-110 cells, RGDS at 500 μg/ml significantly inhibited melanoma cells proliferation induced by human fibroblast growth factor-2 (FGF-2). Furthermore, RGDS treatment (48 h, 500 μg/ml) increased the percentage of cells in sub-G1-phase from 3% (with FGF-2 alone) to 13.2% (with FGF-2 and RGDS), indicating that RGDS might induce apoptosis in melanoma cells, in the presence of FGF-2
在过敏性哮喘的豚鼠模型中,用鼻内滴注的RGD(2.5 mM,200μl)局部治疗气道可减轻过敏原诱导的气道平滑肌增生和过度收缩,以及平滑肌肌球蛋白重链和增殖标志物增殖细胞核抗原的肺表达增加。
In a guinea pig model of allergic asthma, topical treatment of the airways with intranasally instilled RGDS (2.5 mM, 200 μl) attenuated allergen-induced airway smooth muscle hyperplasia and hypercontractility as well as increased pulmonary expression of smooth muscle myosin heavy chain and the proliferative marker proliferating cell nuclear antigen .
Fibronectin is an extracellular matrix protein that plays a critical role in cell adhesion, migration, and differentiation. Fibronectin subunits are composed of repeating units of three types of modules: type I, type II, and type III. The active fragment of fibronectin refers to a small peptide sequence within the type III modules of fibronectin that has been shown to have potent biological activity.\nThe fibronectin active fragment, also known as the cell-binding domain or RGD domain, is a short peptide sequence consisting of the amino acid sequence Arg-Gly-Asp (RGD). This peptide sequence interacts with cell surface receptors known as integrins, which are important for mediating cell adhesion, migration, and signaling.\nThe fibronectin active fragment has been extensively studied as a research tool to investigate the mechanisms of cell adhesion and migration. It has also been used in tissue engineering applications to promote cell attachment and proliferation on synthetic biomaterials. This product is available as a 0.5mg vial.
Fibronectin is an extracellular matrix protein that plays a critical role in cell adhesion, migration, and differentiation. Fibronectin subunits are composed of repeating units of three types of modules: type I, type II, and type III. The active fragment of fibronectin refers to a small peptide sequence within the type III modules of fibronectin that has been shown to have potent biological activity.\nThe fibronectin active fragment, also known as the cell-binding domain or RGD domain, is a short peptide sequence consisting of the amino acid sequence Arg-Gly-Asp (RGD). This peptide sequence interacts with cell surface receptors known as integrins, which are important for mediating cell adhesion, migration, and signaling.\nThe fibronectin active fragment has been extensively studied as a research tool to investigate the mechanisms of cell adhesion and migration. It has also been used in tissue engineering applications to promote cell attachment and proliferation on synthetic biomaterials.
化学预防肽是有助于预防疾病(例如癌症或糖尿病)的发作或发展的肽。这些肽可以源自天然来源,例如大豆或牛奶,也可以来自肽模拟物的设计,也可以源自使用合成肽进行的肽筛选。据认为,这些肽中的某些可以充当细胞周期的调节剂,其调节使细胞通过复制周期前进所需的蛋白质的产生和功能。另外,现在有越来越多的证据表明特定的饮食模式,食物和饮料以及其他饮食物质可以而且确实可以预防癌症。越来越多的流行病学研究表明,食物,营养和身体活动在预防和改变癌症过程中很重要。包括植物蛋白酶抑制剂,乳铁蛋白,乳铁蛋白,凝集素和lunasin在内的不同类型的食物蛋白和多肽似乎起着化学预防剂的作用。如今,蛋白质和多肽被认为是一组营养保健品,在预防癌症的不同阶段(包括起始,促进和进展)方面显示出潜力。此外,已经发现在植物中发现的一些蛋白酶抑制剂,例如豆类和大豆,是有效的癌发生抑制剂。致癌作用是引发和促进癌症的过程。 Bowman-Birk抑制剂和Kunitz胰蛋白酶抑制剂就在其中。目前,这些化合物在致癌作用中的生物学功能主要归因于抑制癌细胞的侵袭和转移,但是,其作用机理仍不完全清楚,需要进一步研究以充分阐明它们。
Definition
The integrins are a superfamily of cell adhesion receptors that bind to extracellular matrix EMC ligands, cell-surface ligands, and soluble ligands.
Discovery
The discovery of integrins was driven in large part by a series of early observation suggesting that adhesion to ECM is mediated at the cell surface receptors. By 1980s, it became clear that fibronectin was one of the groups of ECM proteins present in the serum that could promote the adhesion of cells to the tissue culture flask.
Structure of integrins
The integrins are a family of alpha, beta heterodimeric receptors. Integrins are expressed by all multicellular animals, but their diversity varies widely among species; for example, in mammals, 19 alpha and 8 beta subunit genes encode polypeptides that combine to form 25 different receptors, whereas the Drosophila and Caenorhabditis genomes encode only five and two integrin alpha subunits respectively.
The N-terminal portion of integrin a subunits comprises seven homologous, tandemly repeated domains of about 50 amino acids. Repeats 4-7 (or in some integrins 5-7) contain cation-binding sequences. Seven integrin a subunits (a1, a2, aE, aL, aM, aX, and aD) contain a domain of ~200 amino acids inserted between the second and third N-terminal repeats. This domain is homologous in sequence to the ‘A’ domains of von Willebrand factor, and has been shown to contain a single cation binding site1
The b ?subunit contains a region of ~240 amino acids near its N terminus that is highly conserved between different b subunits. This region may also have an A-domain-like structure with a cation binding site. The C-terminal portion of the b ?subunit contains a number of cysteine-rich repeats2.
Mechanism of action
Cell-cell and cell-substratum adhesion is mediated by the binding of integrin extracellular domains to diverse protein ligands; however, cellular control of these adhesive interactions and their translation into dynamic cellular responses, such as cell spreading or migration, requires the integrin cytoplasmic tails. These short tails bind to intracellular ligands that connect the receptors to signalling pathways and cytoskeletal networks. Hence, by binding both extracellular and intracellular ligands, integrins provide a transmembrane link for the bidirectional transmission of mechanical force and biochemical signals across the plasma membrane. One important mechanism by which cells regulate integrin function is through tight spatial and temporal control of integrin affinity for extracellular ligands. This is achieved by rapid, reversible changes in the conformation of the extracellular domains of the integrin heterodimer, so-called integrin activation3.
Functions
Proliferation: The mechanism by which integrins control proliferation involves both a direct crosstalk between integrins and growth factor receptors GFRs, and GF- independent signalling from integrins themselves. In some cells, for example, ERK signalling is induced directly by integrin adhesion, whereas the Akt pathway (which also promotes proliferation) can be activated downstream of integrins through mechanisms separate to those of GFRs4.
Apoptosis: Integrins are essential determinants of cell survival and, in many cases, prevention or alteration of integrin adhesion triggers a form of apoptosis that is known as anoikis. Anoikis is particularly relevant when cells become located in ECM environments in which they are not developmentally programmed to reside. For example, mammary epithelial cells are normally situated on a laminin-rich basement membrane but, if they are displaced to a stromal ECM of collagen I, they undergo anoikis5.
Differentiation: For some cell types, the involvement of integrins during their developmental programming to become fully mature, differentiated cells has been extensively characterized. Oligodendrocyte differentiation is a particularly neat example, because two integrin-GFR switches occur. In the first switch, PDGFaR collaborates with the vitronectin receptor avb3 integrin to promote proliferation of oligodendrocytes but, upon contact between cell processes and laminin-2, the same GFR (now in lipid rafts) works with a6b1 integrin to send survival signals. In the second switch, the EGF-family protein neuregulin sends survival and proliferation signals in oligodendrocyte precursors but, upon contact with laminin-2, the neuregulin receptors ErbB2 and ErbB4 provide signals that promote oligodendrocyte differentiation instead6.
Integrins control the cell-division axis: In studies conducted with conventional 2D tissue culture, cells divide in the plane of the dish to which they adhere, requiring alignment of the mitotic spindle parallel to the substratum (in the xy direction). Using micro-patterned ECM substrata that allow single cells to adhere with specific topologies, it has been shown that the tension exerted by the ECM (through integrin-containing adhesion) generates defined actin-cytoskeleton force fields within cells during interphase. During metaphase, cells round up to undergo mitosis, but retraction fibres transfer the polarity of tension that was established in interphase to the astral microtubules (which link the spindle poles with the cell cortex and, therefore, the plasma membrane), thereby aligning the mitotic spindle. Retraction fibres are bound to the substratum by integrins, so cell-matrix adhesion determines the internal architecture of cells, which subsequently defines the spindle position and, therefore, the division axis at mitosis7.
References
1. Michishita, M, Videm, V. and Arnaout, MA (1993). A novel divalentcation binding site in the A domain of the a integrin CR3 (CD11b/CD18) is essential for ligand binding. Cell., 72: 857-867.
2. Lee OJ, Rieu, Arnaout MA and Liddington, R. (1995a). Crystal structure of the A-domain from the b ?subunit of the integrin CR3 (CD11a/CD18). Cell., 80: 631-638.
3. Woodside DG, Liu S and Ginsberg MH (2001). Integrin activation. Thromb. Haemost., 86, 316-323.
4. Velling T, Stefansson A and Johansson S (2008). EGFR and beta1 integrins utilize different signaling pathways to activate Akt. Exp. Cell Res., 314: 309-316.
5. Pullan S, Wilson J, Metcalfe A, Edwards GM, Goberdhan N, Tilly J, Hickman JA, Dive C. and Streuli CH (1996). Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J. Cell Sci., 109: 631- 642.
6. Baron W, Colognato H and ffrench-Constant C (2005). Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia., 49: 467- 479.
7. Thery, M, Racine V, Pepin A, Piel M, Chen Y , Sibarita JB and Bornens M (2005). The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol., 7: 947-953.

RGD肽-说明
RGD肽是指含有由Arg-Gly-Asp三个氨基酸组成的序列多肽,有直线肽和环肽之分。它们是许多细胞外基质蛋白(如VN、FN、FGN、胶原等)等最小识别短肽序列。

研究发现,RGD序列肽具有广泛的生物活性,可用于心血管疾病、骨质疏松和炎症等疾病的治疗,还可以预防和治疗由细胞粘附异常而导致的肿瘤,尤其是发展性肿瘤的转移;另一方面,RGD 序列肽又可作为兴奋剂,促进损伤的器官与组织的再生、伤口的愈合等等,RGD作为某些整合素的受体,其选择性部分依赖于RGD的构象以及RGD周围的氨基酸残基。

为此,近几年,许多科技工作者合成了一系列RGD三肽、四肽、五肽等,还合成了RGD环肽、双线肽、RGD模拟肽等等。为了满足客户对各种RGD序列肽的需求,专肽生物提供最广泛的RGD序列肽库,以满足科研工作者对RGD肽的需求。
专肽生物提供各种RGD肽的现货,缩短科研工作者的项目时间,例如c(RGDfK)、c(RGDfC)、c(RADyK)、c(RGDyK)、c(RADfC)、环状多肽c(RGDfK)-巯基乙酸、c(RGDfK)-PEG2-巯基乙酸、Mpa-Ahx-c(RGDfK)、环状多肽c(RGDfK)-半胱氨酸、DOTA-c(RGDfK)、NOTA-c(RGDfK)、NOTA-c(RGDyK)、DOTA-c(RGDyK)、E[c(RGDfK)]2、E[c(RGDyK)]2、DDDDD-c(RGDfK)等等,具体可咨询销售人员。
| DOI | 名称 | |
|---|---|---|
| 10.1186/1476-4598-9-84 | Intracellular targets of RGDS peptide in melanoma cells | 下载 |
| 10.1164/rccm.200907-1065OC | The integrin-blocking peptide RGDS inhibits airway smooth muscle remodeling in a guinea pig model of allergic asthma | 下载 |





