400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研
Definition
Dodecapeptide tachykinin that is kassinin is found in the central nervous system of the amphibian Kassina senegalensis. It is similar in structure and action to other tachykinins, but it is especially effective in contracting smooth muscle tissue and stimulating the micturition reflex.
Discovery
In 1964, Erspamer et al., first demonstrated the occurrence of bioactive peptide, kassinin in amphibian skin1. A biosynthetic precursor of kassinin cDNA encoding the novel kassinin analog (Thr2, Ile9)-kassinin was identified in skin secretion of amphibian2. In 1983, two new mammalian tachykinins, neurokinin A and neurokinin B, were discovered in the porcine spinal cord. Their pharmacological actions more closely resemble those of the amphibian tachykinin kassinin and the molluscan tachykinin eledoisin3.
Structural Characteristics
Tachykinins are among the most widely-studied families of regulatory peptides characterized by a highly-conserved C-terminal -Phe-X-Gly-Leu-Met amide motif, which also constitutes the essential bioactive core. Both the aqueous and lipid-induced structure of kassinin, has been studied by Rani et al., (2001). Water kassinin prefers to be in an extended chain conformation, in the presence of perdeuterated dodecylphosphocholine (DPC) micelles, a membrane model system, helical conformation is induced in the central core and C-terminal region (K4-M12) of the peptide. N-terminus though less defined also displays some degree of order and a possible turn structure. The conformation adopted by kassinin in the presence of DPC micelles is consistent with the structural motif typical of neurokinin-1 selective agonists and with that reported for eledoisin in hydrophobic environment4.
Mode of Action
In frog skin, tachykinins stimulate the ion transport, by interacting with NK1-like receptors which can be estimated by measuring the short-circuit current (SCC) value. Kassinin (NK2 preferring in mammals) increases the SCC5. Kassinin also induces concentration-related contractions of the longitudinal muscle of the mouse distal colon. Contractile responses to the tachykinins result from a direct activation of smooth muscle cells. Kassinin evokes a contractile response in the absence of external Ca2+ and their myogenic activity was, to some extent, resistant to the inhibitory effect of nifedipine (a calcium channel blocker). So an additional process, probably the release of an intracellularly bound Ca2+ store, participates in the mechanism by which kassinin contracts the mouse distal colon. After desensitization of the mouse distal colon to Substance P (SP), the contractile activity provoked by SP was totally abolished whilst the responses evoked by kassinin were barely affected. These observations and other experimental findings indirectly support the assumption that the mouse distal colons possess different tachykinin-binding sites6.
Functions
Effect on rat urinary bladder - Synthetic replicates of kassinin are found to be active on rat urinary bladder smooth muscle at nanomolar concentrations2. Kassinin induces concentration-related contractions of the longitudinal muscle of the mouse distal colon.
Effect on endocrine pancreatic function - The effect of kassinin on endocrine pancreatic function was examined in the rat. Kassinin, injected intravenously in graded doses 10, 20, and 30 min before blood collection, significantly increased both plasma insulin and plasma glucagon in a dose-related fashion. The largest dose examined (10 µg) increased plasma insulin by 275% and plasma glucagon by 77% 7.
Synthetic kassinin affects splanchnic circulation - Effects of intravenously administered synthetic kassinin on splanchnic circulation and exocrine pancreatic secretion was examined in six anesthetized dogs. Kassinin caused dose-related increases in the blood flow in superior mesenteric artery and portal vein, and produced an initial increase followed by a decrease in pancreatic blood flow, but did not affect the exocrine pancreatic secretion. This study suggests that kassinin functions as a neuropeptide controlling the splanchnic circulation in mammalian species8.
References
1. Book : Handbook of chemical neuroanatomy. Chapter VI Neurokinin receptors in the CNS by Da-silva R, Mcleod AL, Krause JE.
2. Wang L, Zhou M, Lynch L, Chen T, Walker B, Shaw C (2009). Kassina senegalensis skin tachykinins: Molecular cloning of kassinin and (Thr2, Ile9)-kassinin biosynthetic precursor cDNAs and comparative bioactivity of mature tachykinins on the smooth muscle of rat urinary bladder. Biochimie, 91(5): 613-619.
3. Tan DP and Tsou K (1988). Differential Effects of Tachykinins Injected Intranigrally on Striatal Dopamine Metabolism. Journal of Neurochemistr, 51(5): 1333-1337.
4. Rani CR, Lynn AM, Cowsik SM (2001). Lipid Induced Conformation of the Tachykinin Peptide Kassinin. Journal of Biomolecular Structure and Dynamics, 18 (4): 611-625.
5. Lippe C, Bellantuon V, Ardizzone C, Cassano G (2004). Eledoisin and Kassinin, but not Enterokassinin, stimulate ion transport in frog skin. Peptides, 25(11): 1971-1975.
6. Fontaine J and Lebrun P (1989). Contractile effects of substance P and other tachykinins on the mouse isolated distal colon. Br J Pharmacol, 96(3): 583–590.
7. Gullner HG, Yajimsa H, Harris V, Unger RH (1982). Kassinin: Stimulation of Insulin and Glucagon Secretion in the Rat. Endocrinology, 110 (4): 1246-1248.
8. Doi R, Inoue K, Kogire M, Sumi S, Takaori K, Yun M, Yajima H, Tobe T (1988). Effects of synthetic kassinin on splanchnic circulation and exocrine pancreas in dogs. Peptides, 9(5): 1055-1058.
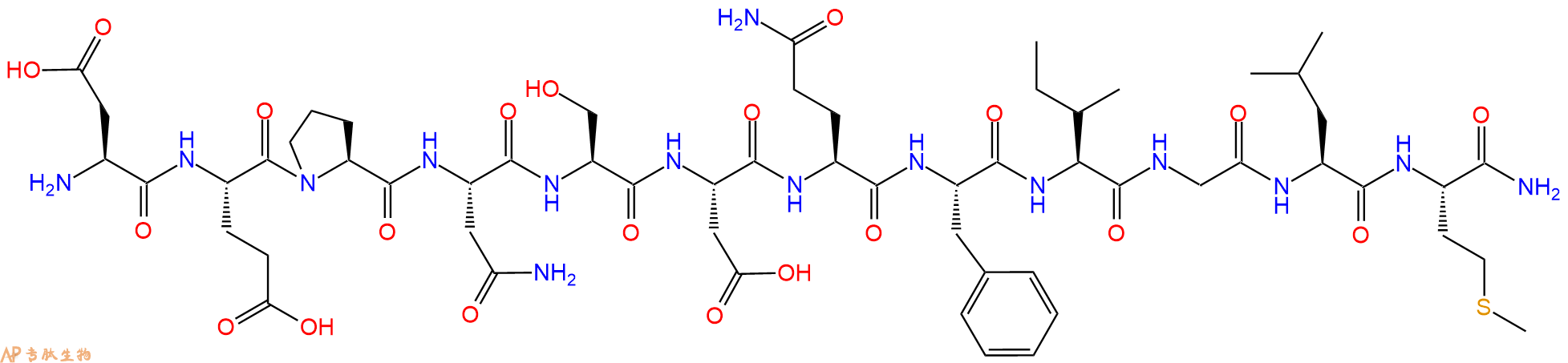
多肽H2N-Asp-Glu-Pro-Asn-Ser-Asp-Gln-Phe-Ile-Gly-Leu-Met-NH2的合成步骤:
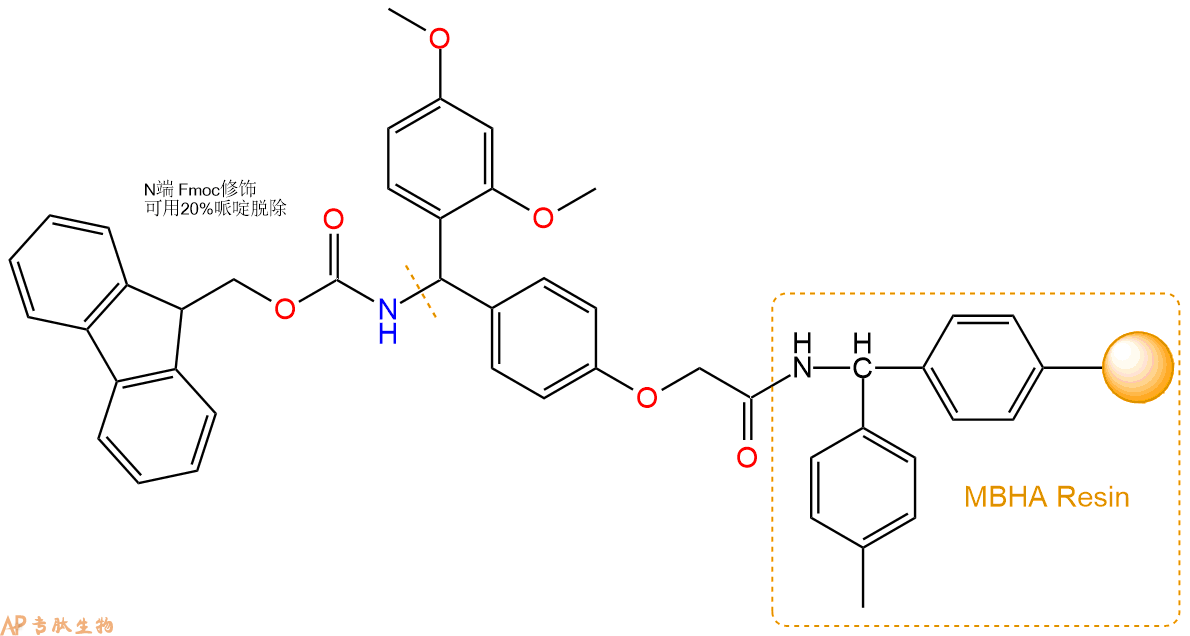
1、合成MBHA树脂:取若干克的MBHA树脂(如初始取代度为0.5mmol/g)和1倍树脂摩尔量的Fmoc-Linker-OH加入到反应器中,加入DMF,搅拌使氨基酸完全溶解。再加入树脂2倍量的DIEPA,搅拌混合均匀。再加入树脂0.95倍量的HBTU,搅拌混合均匀。反应3-4小时后,用DMF洗涤3次。用2倍树脂体积的10%乙酸酐/DMF 进行封端30分钟。然后再用DMF洗涤3次,甲醇洗涤2次,DCM洗涤2次,再用甲醇洗涤2次。真空干燥12小时以上,得到干燥的树脂{Fmoc-Linker-MHBA Resin},测定取代度。这里测得取代度为 0.3mmol/g。结构如下图:
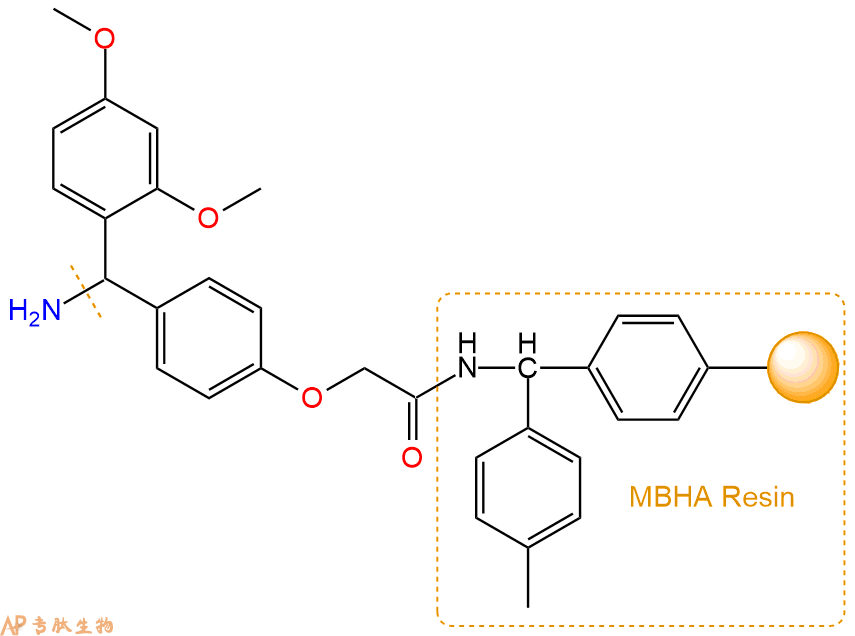
2、脱Fmoc:取1.96g的上述树脂,用DCM或DMF溶胀20分钟。用DMF洗涤2遍。加3倍树脂体积的20%Pip/DMF溶液,鼓氮气30分钟,然后2倍树脂体积的DMF 洗涤5次。得到 H2N-Linker-MBHA Resin 。(此步骤脱除Fmoc基团,茚三酮检测为蓝色,Pip为哌啶)。结构图如下:
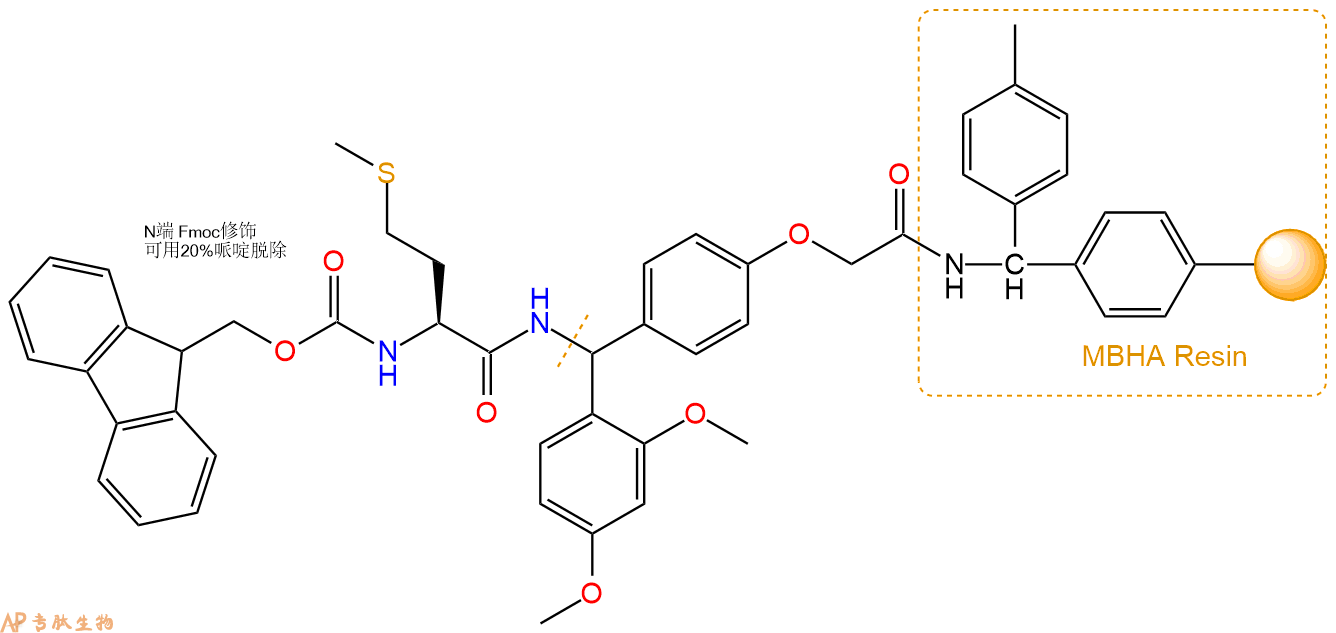
3、缩合:取1.76mmol Fmoc-Met-OH 氨基酸,加入到上述树脂里,加适当DMF溶解氨基酸,再依次加入3.53mmol DIPEA,1.68mmol HBTU。反应30分钟后,取小样洗涤,茚三酮检测为无色。用2倍树脂体积的DMF 洗涤3次树脂。(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。得到Fmoc-Met-Linker-MBHA Resin。氨基酸:DIPEA:HBTU:树脂=3:6:2.85:1(摩尔比)。结构图如下:
4、依次循环步骤二、步骤三,依次得到
H2N-Met-Linker-MBHA Resin
Fmoc-Leu-Met-Linker-MBHA Resin
H2N-Leu-Met-Linker-MBHA Resin
Fmoc-Gly-Leu-Met-Linker-MBHA Resin
H2N-Gly-Leu-Met-Linker-MBHA Resin
Fmoc-Ile-Gly-Leu-Met-Linker-MBHA Resin
H2N-Ile-Gly-Leu-Met-Linker-MBHA Resin
Fmoc-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin
H2N-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin
Fmoc-Gln(Trt)-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin
H2N-Gln(Trt)-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin
Fmoc-Asp(OtBu)-Gln(Trt)-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin
H2N-Asp(OtBu)-Gln(Trt)-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin
Fmoc-Ser(tBu)-Asp(OtBu)-Gln(Trt)-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin
H2N-Ser(tBu)-Asp(OtBu)-Gln(Trt)-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin
Fmoc-Asn(Trt)-Ser(tBu)-Asp(OtBu)-Gln(Trt)-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin
H2N-Asn(Trt)-Ser(tBu)-Asp(OtBu)-Gln(Trt)-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin
Fmoc-Pro-Asn(Trt)-Ser(tBu)-Asp(OtBu)-Gln(Trt)-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin
H2N-Pro-Asn(Trt)-Ser(tBu)-Asp(OtBu)-Gln(Trt)-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin
Fmoc-Glu(OtBu)-Pro-Asn(Trt)-Ser(tBu)-Asp(OtBu)-Gln(Trt)-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin
H2N-Glu(OtBu)-Pro-Asn(Trt)-Ser(tBu)-Asp(OtBu)-Gln(Trt)-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin
Fmoc-Asp(OtBu)-Glu(OtBu)-Pro-Asn(Trt)-Ser(tBu)-Asp(OtBu)-Gln(Trt)-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin
以上中间结构,均可在专肽生物多肽计算器-多肽结构计算器中,一键画出。
最后再经过步骤二得到 H2N-Asp(OtBu)-Glu(OtBu)-Pro-Asn(Trt)-Ser(tBu)-Asp(OtBu)-Gln(Trt)-Phe-Ile-Gly-Leu-Met-Linker-MBHA Resin,结构如下:
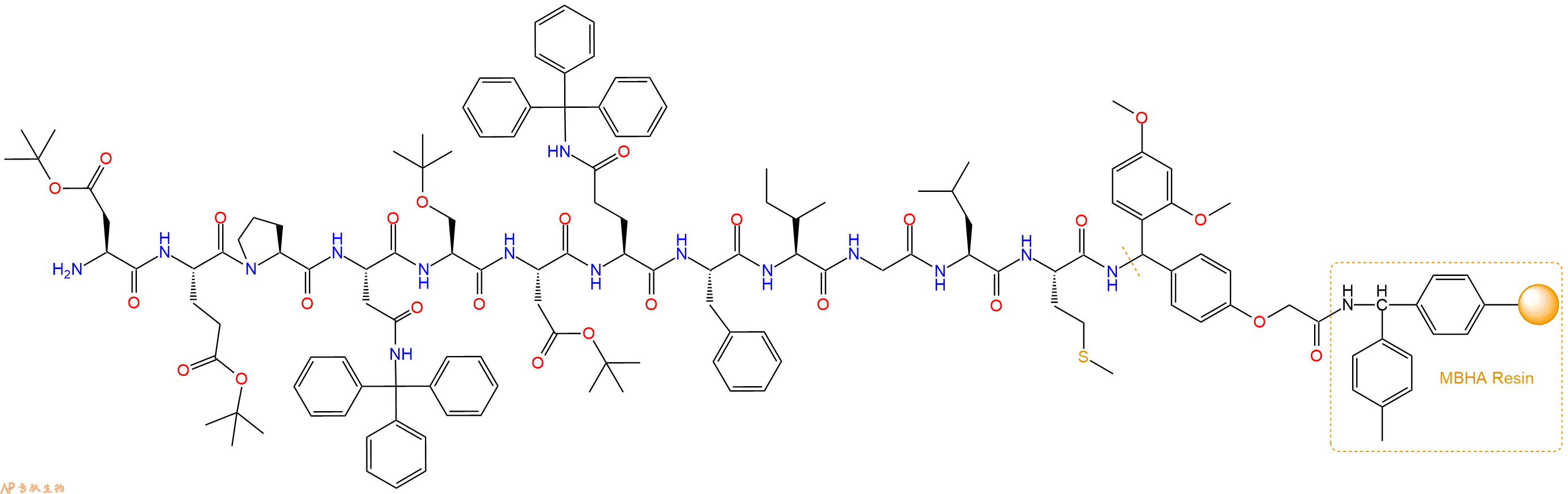
5、切割:6倍树脂体积的切割液(或每1g树脂加8ml左右的切割液),摇床摇晃 2小时,过滤掉树脂,用冰无水乙醚沉淀滤液,并用冰无水乙醚洗涤沉淀物3次,最后将沉淀物放真空干燥釜中,常温干燥24小试,得到粗品H2N-Asp-Glu-Pro-Asn-Ser-Asp-Gln-Phe-Ile-Gly-Leu-Met-NH2。结构图见产品结构图。
切割液选择:1)TFA:H2O=95%:5%
2)TFA:H2O:TIS=95%:2.5%:2.5%
3)三氟乙酸:茴香硫醚:1,2-乙二硫醇:苯酚:水=87.5%:5%:2.5%:2.5%:2.5%
(前两种适合没有容易氧化的氨基酸,例如Trp、Cys、Met。第三种适合几乎所有的序列。)
6、纯化冻干:使用液相色谱纯化,收集目标峰液体,进行冻干,获得蓬松的粉末状固体多肽。不过这时要取小样复测下纯度 是否目标纯度。
7、最后总结:
杭州专肽生物技术有限公司(ALLPEPTIDE https://www.allpeptide.com)主营定制多肽合成业务,提供各类长肽,短肽,环肽,提供各类修饰肽,如:荧光标记修饰(CY3、CY5、CY5.5、CY7、FAM、FITC、Rhodamine B、TAMRA等),功能基团修饰肽(叠氮、炔基、DBCO、DOTA、NOTA等),同位素标记肽(N15、C13),订书肽(Stapled Peptide),脂肪酸修饰肽(Pal、Myr、Ste),磷酸化修饰肽(P-Ser、P-Thr、P-Tyr),环肽(酰胺键环肽、一对或者多对二硫键环),生物素标记肽,PEG修饰肽,甲基化修饰肽等。
以上所有内容,为专肽生物原创内容,请勿发布到其他网站上。





