400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研
抗菌肽和蜂毒素都具有抗菌效果。抗菌肽A与蜂毒素A组成而成的杂合肽,具有很强的抑菌活性。抗菌肽中一般含有比较多的碱性氨基酸,因此亲水性是比较强的,这部分是抗菌肽的生物活性片段;而蜂毒素独特的疏水性以及抗菌活性,可以让多肽进入细菌质膜中,两种片段结合,能够起到消灭细菌的效果,Cecropin A (1-7)-Melittin A (2-9) amide就是这样一条具有灭菌效果的杂合肽,由15个氨基酸组合而成
编号:119043
CAS号:157606-25-2
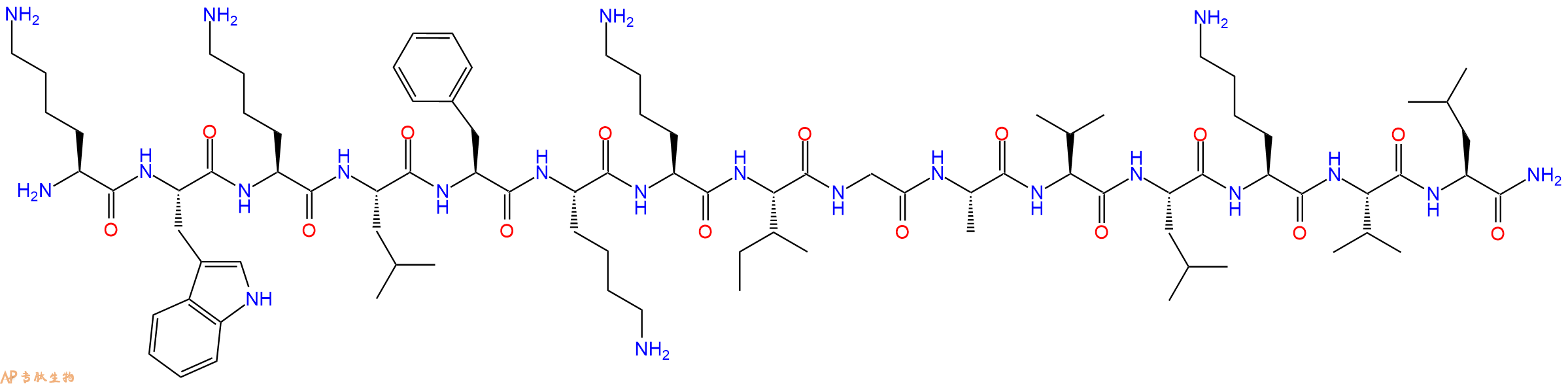
单字母:H2N-KWKLFKKIGAVLKVL-CONH2
| 参考文献(References): | D. Andreau et al., PNAS, 80, 6475 (1983) D. Andreau et al., Biochem., 24, 1683 (1985) |
抗菌肽A(1-7)-蜂毒肽A(2-9)酰胺,也称为CAMEL0,是一种合成的杂合肽,由天然抗生素肽抗菌肽A和蜂毒肽的一部分组成。CAMEL0显示出比天然分子更好的抗菌活性,但缺乏蜂毒肽的溶血特性。研究表明,其抗菌活性范围不仅限于需氧微生物,还包括几种革兰氏阴性和革兰氏阳性厌氧微生物。通过其确定的广谱抗生素活性,这种杂合肽也可能是环丙沙星治疗炭疽感染的有效替代品。
Cecropin A (1-7)-Melittin A (2-9) amide, also referred to as CAMEL0, is a synthetic hybrid peptide that is composed of portions of the naturally occurring antibiotic peptide cecropin A and melittin. CAMEL0 shows a better antimicrobial activity than the native molecules, but lacks the hemolytic properties of melittin. Studies revealed that the range of its antimicrobial activity is not only restricted to aerobic microorganisms but also included several gram-negative and gram-positive anaerobic microorganisms. Through its ascertained broad spectrum of antibiotic activity, this hybrid peptide may also represent an effective substitute for ciprofloxacin in the treatment of anthrax infections.
抗菌肽和蜂毒素都具有抗菌效果。抗菌肽A与蜂毒素A组成而成的杂合肽,具有很强的抑菌活性。抗菌肽中一般含有比较多的碱性氨基酸,因此亲水性是比较强的,这部分是抗菌肽的生物活性片段;而蜂毒素独特的疏水性以及抗菌活性,可以让多肽进入细菌质膜中,两种片段结合,能够起到消灭细菌的效果,Cecropin A (1-7)-Melittin A (2-9) amide就是这样一条具有灭菌效果的杂合肽,由15个氨基酸组合而成
A 15-residue hybrid peptide incorporating partial sequences of cecropin A and melittin that causes the release of carboxyfluoresceine encapsulated in phosphatidylcholine liposomes.
抗菌肽A(1-7)-蜂毒肽A(2-9)酰胺三氟乙酸盐是一种固定在纤维素纳米纤维上的抗菌肽。它已被证明对枯草芽孢杆菌具有很强的抗菌活性,固定化过程确保肽不会从材料表面释放出来。用纳米纸涂覆然后施加肽的过程提供了具有高抗菌活性的稳定表面。使用Fmoc化学通过固相合成合成合成肽,并通过制备型HPLC纯化。
Cecropin A (1-7)-Melittin A (2-9) amide trifluoroacetate salt is a cecropin-melittin hybrid peptide that has been immobilized on cellulose nanofibers for use as an antimicrobial. It has been shown to have strong antimicrobial activity against bacillus subtilis, and the immobilization process ensures that the peptide is not released from the surface of the material. The process of coating with nanopaper and then applying the peptides provides a stable surface with high antimicrobial activity. The synthetic peptides are synthesized by solid phase synthesis using Fmoc chemistry and purified by preparative HPLC.
一种由15个残基组成的混合肽,含有天蚕素A和蜂毒肽的部分序列,可释放包裹在磷脂酰胆碱脂质体中的羧基荧光素。
A 15-residue hybrid peptide incorporating partial sequences of cecropin A and melittin that causes the release of carboxyfluoresceine encapsulated in phosphatidylcholine liposomes.
| DOI | 名称 | |
|---|---|---|
| 10.1016/j.peptides.2014.09.021 | Inhibition and destruction of Pseudomonas aeruginosa biofilms by antibiotics and antimicrobial peptides | 下载 |





