400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研
一种致脑肽,可诱导碱性蛋白特异性 T 细胞增殖。在外周血单核细胞中引起 Th1 极化,与多发性硬化症 (MS) 有关。
编号:172728
CAS号:118506-26-6
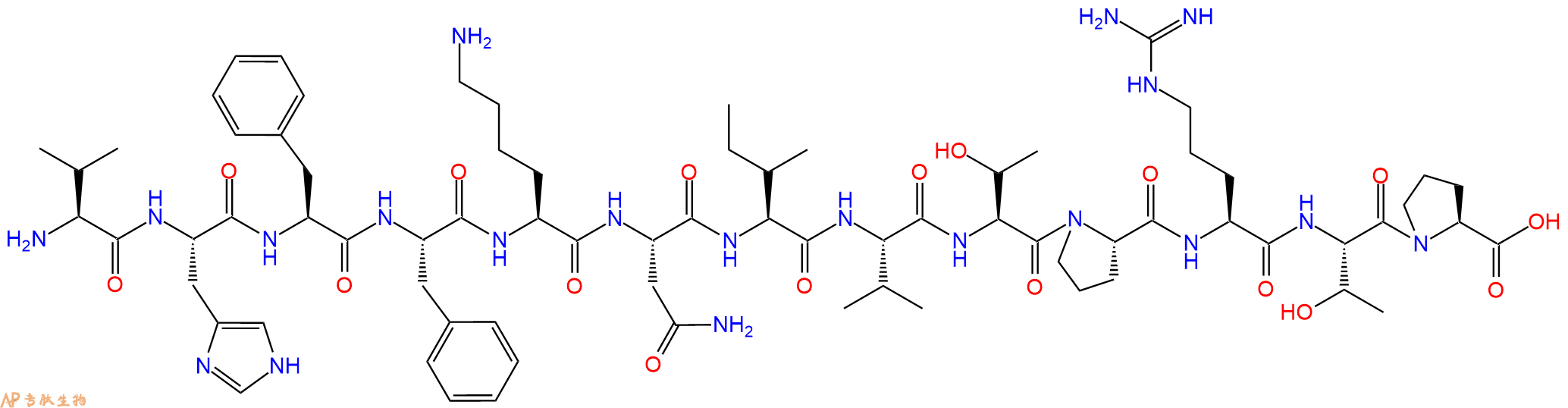
单字母:H2N-VHFFKNIVTPRTP-OH
| 参考文献(References): | R.E.Jones et al., J. Neuroimmunol., 37, 203 (1992) |
Myelin Basic Protein(87-99) TFA 是一种致脑肽,可诱导碱性蛋白特异性 T 细胞增殖。Myelin Basic Protein(87-99) TFA 在外周血单核细胞中引起 Th1 极化,与多发性硬化症 (MS) 有关。
Myelin Basic Protein(87-99) TFA is an encephalitogenic peptide that induces basic protein-specific T cell proliferation. Myelin Basic Protein(87-99) TFA causes a Th1 polarization in peripheral blood mononuclear cells with is implicated of multiple sclerosis (MS).
髓磷脂碱性蛋白(87-99)醋酸盐是一种致脑肽,在中枢神经系统中诱导T细胞增殖和Th1极化。髓磷脂碱性蛋白(MBP)是一种潜在的靶抗原,因为它在易感动物中诱导实验性变态反应性脑脊髓炎(EAE)。
Myelin Basic Protein (87-99) acetate is an encephalitogenic peptide that induces T cell proliferation with Th1 polarization in the CNS. Myelin basic protein (MBP) is a potential target antigen because it induces experimental allergic encephalomyelitis (EAE) in susceptible animals.
这是髓磷脂碱性蛋白(MBP)的片段,对应于小鼠序列的氨基酸84-96(豚鼠86-98;人类87-99)。
This is fragment of the myelin basic protein (MBP), which corresponds to amino acids 84-96 of the murine sequence (86-98 in guinea pig; 87-99 in human).
髓鞘位于中枢神经系统(CNS)和周围神经系统中,对于神经绝缘和神经冲动的有益传导至关重要。当髓鞘被破坏时,神经变性和传导失败就会发生。这可以在中枢神经系统的脱髓鞘疾病中观察到,例如:急性播散性脑脊髓炎和多发性硬化症,以及在PNS中:格林-巴雷综合征和Charcot-Marie-Tooth病。衍生该产物的nMyelin碱性蛋白(MBP)是髓鞘中第二丰富的蛋白质。已发现它是一种内在无序的蛋白质,根据环境条件,它可以改变其构象。它还折叠成⍺-螺旋结构,使MBP与脂质双层表面紧密结合。MBP还与其他蛋白质相互作用,即细胞骨架蛋白和钙调蛋白,并可能参与信号传导途径。尽管需要进行更多的研究,但认为MBP对多发性硬化症的发病机制有显着贡献。由于MBP是一种自身抗原,因此它可以被自身抗体识别和切割,并且是免疫蛋白酶体的底物。另外的研究发现,MBP的翻译后修饰(例如精氨酸的去除)增加,并可能参与多发性硬化症的发病机制。因此,这种源自MBP的蛋白质可用于在研究和动物模型中模拟神经退行性疾病的表型。
The myelin sheath which is located in both the Central Nervous System (CNS) and the Peripheral Nervous System is crucial for neural insulation and the salutatory conduction of nerve impulses. When this myelin sheath is destroyed neurodegeneration and conduction failure occur. This can be observed in demyelinating diseases in the CNS such as: acute disseminated encephalomyelitis and multiple sclerosis and within the PNS: Guillain–Barré syndrome and Charcot–Marie–Tooth disease.Myelin Basic Protein (MBP) from which this product is derived is the second most abundant protein in myelin. It has been found to be an intrinsically disordered protein and depending on the environmental conditions it can change its conformation. It also folds into ⍺-helical structures which allow MBP to bind tightly to lipid bilayer surfaces. MBP also interacts with other proteins, namely cytoskeletal proteins and calmodulin and may be involved in signalling pathways.Although more research needs to be carried out, it is thought that MBP significantly contributes to the pathogenesis of multiple sclerosis. As MBP is an autoantigen it can be recognized and cleaved by autoantibodies and is a substrate for the immunoproteasome. Additional research has found that post-translational modifications of MBP such as the removal of arginine are increased in and may be involved in the pathogenesis of multiple sclerosis. Therefore this protein derived from MBP can be used to mimic Neurodegenerative disease phenotypes in research and animal models.
该肽是髓鞘碱性蛋白的免疫显性肽,已显示其诱导致脑炎性髓鞘碱性蛋白特异性T细胞系的增殖。用该肽主动免疫大鼠诱导急性实验性自身免疫性脑脊髓炎。该蛋白质区域在哺乳动物物种中高度保守。
This peptide is the immunodominant eptitope of Myelin Basic Protein, which has been shown to induce the proliferation of an encephalitogenic, myelin basic protein-specific T cell line. Active immunization of rats with this peptide induced acute experimental autoimmune encephalomyelitis. This protein region is highly conserved among mammalian species.
R E Jones, et al. The synthetic 87-99 peptide of myelin basic protein is encephalitogenic in Buffalo rats. J Neuroimmunol. 1992 Apr;37(3):203-12. : https://pubmed.ncbi.nlm.nih.gov/1373154
George Deraos, et al. Citrullination of linear and cyclic altered peptide ligands from myelin basic protein (MBP(87-99)) epitope elicits a Th1 polarized response by T cells isolated from multiple sclerosis patients: implications in triggering disease. J Med Chem. 2008 Dec 25;51(24):7834-42. : https://pubmed.ncbi.nlm.nih.gov/19053745





