400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研
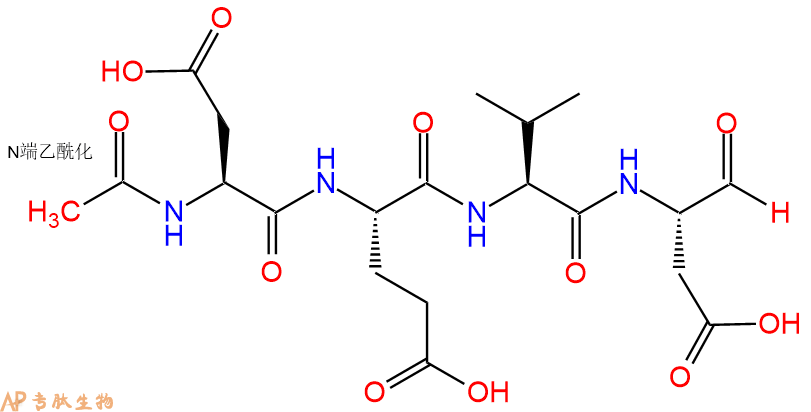
通过caspase-3(IC 50 = 0.2nM)切割聚(ADP-核糖)聚合酶(PARP)的有效抑制剂。
编号:194344
CAS号:184179-08-6/169332-60-9
单字母:Ac-DEVD-CHO
| 编号: | 194344 |
| 中文名称: | Caspase 3 (Apopain) Inhibitor 1 |
| 英文名: | Caspase 3 (Apopain) Inhibitor 1 |
| 英文同义词: | PASE-3 Inhibitor I、Caspase-3 Inhibitor I |
| CAS号: | 184179-08-6/169332-60-9 |
| 单字母: | Ac-DEVD-CHO |
| 三字母: | Ac N端乙酰化封端,一种常见的修饰方式,常用于模拟蛋白质中的肽片段。 -AspL-天冬氨酸:aspartic acid。系统命名为(2S)-氨基-丁二酸。是编码氨基酸,又是神经递质。符号:D,Asp。D-天冬氨酸存在于多种细菌的细胞壁和短杆菌肽A中。 -GluL-谷氨酸:glutamic acid。系统命名为(2S)-氨基-戊二酸。是编码氨基酸。符号:E,Glu。D-谷氨酸存在于多种细菌的细胞壁和某些细菌杆菌肽中。 -ValL-缬氨酸:valine。系统命名为(2S)-氨基-3-甲基丁酸。是编码氨基酸。是哺乳动物的必需氨基酸。符号:V,Val。在某些放线菌素如缬霉素中存在 D-缬氨酸。 -AspL-天冬氨酸:aspartic acid。系统命名为(2S)-氨基-丁二酸。是编码氨基酸,又是神经递质。符号:D,Asp。D-天冬氨酸存在于多种细菌的细胞壁和短杆菌肽A中。 -CHOC端醛基化,醛基亦称甲酰基。 |
| 氨基酸个数: | 4 |
| 分子式: | C20H30O11N4 |
| 平均分子量: | 502.47 |
| 精确分子量: | 502.19 |
| 等电点(PI): | - |
| pH=7.0时的净电荷数: | -3 |
| 平均亲水性: | 1.875 |
| 疏水性值: | -1.58 |
| 消光系数: | - |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 盐体系: | TFA盐 |
| 生成周期: | 当日发货 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 细胞凋亡肽(Apoptosis Peptides) 半胱氨酸蛋白酶(Caspase) 醛肽 抑制剂相关肽(Inhibitor Peptide) |
| 参考文献(References): | D. Wissing et al., Proc. Natl. Acad. Sci. USA, 94, 5973 (1997) |
Ac-DEVD-CHO是胱天蛋白酶-3(IC)切割聚ADP核糖聚合酶(PARP)的有效抑制剂₅₀ = 0.2nM)。PARP切割发生在细胞凋亡开始时。肽醛能够在体外减弱细胞凋亡事件(IC₅₀ = 10至100nM)。
Ac-DEVD-CHO is a potent inhibitor of the poly(ADP-ribose) polymerase (PARP) cleavage by caspase-3 (IC₅₀ = 0.2 nM). PARP cleavage occurs at the onset of apoptosis. The peptide aldehyde was able to attenuate apoptotic events in vitro (IC₅₀ = 10 to 100 nM).
Ac-DEVD CHO-醋酸盐是一种特异性的半胱氨酸蛋白酶-3抑制剂。
Ac-DEVD-CHO acetate is a specific inhibitor of Caspase-3.
Ac-DEVD-CHO是II组胱天蛋白酶的有效醛抑制剂,胱天蛋白酶-3和胱天蛋白酶-7的Ki值分别为0.2nM和0.3nM。对胱天蛋白酶2的弱抑制作用。
Ac-DEVD-CHO is a potent aldehyde inhibitor of Group II caspases with Ki values of 0.2 nM and 0.3 nM for for caspase-3 and caspase-7, respectively. Weak inhibition for caspase-2.
Ac-Asp-Glu-Val-Asp-H(醛)是属于配体组的肽。它已被用作离子通道的抑制剂和细胞生物学研究的抗体。Ac-Asp-Glu-Val-Asp-H(醛)是IB类代谢型谷氨酸受体的有效激活剂,已被证明可抑制大鼠脑突触体中Na+/K+ATPase泵的活性。该肽还以高亲和力与β2肾上腺素能受体结合,但不激活它们。
Ac-Asp-Glu-Val-Asp-H (aldehyde) is a peptide that belongs to the group of ligands. It has been used as an inhibitor of ion channels and as an antibody for cell biology research. Ac-Asp-Glu-Val-Asp-H (aldehyde) is a potent activator of the class IB metabotropic glutamate receptors and has been shown to inhibit the activity of the Na+/K+ ATPase pump in rat brain synaptosomes. This peptide also binds to beta 2 adrenergic receptors with high affinity, but does not activate them.
caspase-3切割聚(ADP-核糖)聚合酶(PARP)的有效抑制剂(IC50=0.2 nM)。PARP切割发生在细胞凋亡开始时。该肽醛可用于减弱体外凋亡事件(IC50=10 nM)。
Potent inhibitor for the poly(ADP-ribose) polymerase (PARP) cleavage by caspase-3 (IC50=0.2 nM). PARP cleavage occurs at the onset of apoptosis. This peptide aldehyde is useful in attenuating apoptotic events in vitro (IC50 = 10 nM).
Definition
Apoptosis or programmed cell death is a normal component of the development and health of multicellular organisms. Cells die in response to a variety of stimuli and during apoptosis they do so in a controlled, regulated fashion.
Discovery
In 1885, Flemming W described the process of programmed cell death. John Kerr's discovery, in late 1960s, initially called "shrinkage necrosis" but which he later renamed "apoptosis", came about when his attention was caught by a curious form of liver cell death during his studies of acute liver injury in rats 1,2. Kerr in 1972 proposed the term apoptosis is for mechanism of controlled cell deletion, which appears to play a complementary but opposite role to mitosis in the regulation of animal cell populations. Its morphological features suggest that it is an active, inherently programmed phenomenon, and it has been shown that it can be initiated or inhibited by a variety of environmental stimuli, both physiological and pathological 3.
Structural Characteristics
Heterodimerization between members of the Bcl-2 family of proteins is a key event in the regulation of programmed cell death. The molecular basis for heterodimer formation was investigated by determination of the solution structure of a complex between the survival protein Bcl-xL and the death-promoting region of the Bcl-2-related protein Bak. The structure and binding affinities of mutant Bak peptides indicate that the Bak peptide adopts an amphipathic helix that interacts with Bcl-xL through hydrophobic and electrostatic interactions. Mutations in full-length Bak that disrupt either type of interaction inhibit the ability of Bak to heterodimerize with Bcl-xL 4.
The structure of the 16–amino acid peptide complexed with a biologically active deletion mutant of Bcl-xL was determined by nuclear magnetic resonance spectroscopy (NMR). The structure was determined from a total of 2813 NMR-derived restraints and is well defined by the NMR data. The Bak peptide forms a helix when complexed to Bcl-xL. The COOH terminal portion of the Bak peptide interacts predominantly with residues in the BH2 and BH3 regions. Melanoma inhibitor of apoptosis (ML-IAP) is a potent anti-apoptotic protein that is upregulated in a number of melanoma cell lines but not expressed in most normal adult tissues. Overexpression of IAP proteins, such as ML-IAP or the ubiquitously expressed X-chromosome-linked IAP (XIAP), in human cancers has been shown to suppress apoptosis induced by a variety of stimuli. X-ray crystal structures of ML-IAP-BIR in complex with Smac- and phage-derived peptides, together with peptide structure−activity-relationship data, indicate that the peptides can be modified to provide increased binding affinity and selectivity for ML-IAP-BIR relative to XIAP-BIR3 5.
Mode of Action
Upon receiving specific signals instructing the cells to undergo apoptosis a number of distinctive changes occur in the cell. Families of proteins known as caspases are typically activated in the early stages of apoptosis. These proteins breakdown or cleave key cellular components that are required for normal cellular function including structural proteins in the cytoskeleton and nuclear proteins such as DNA repair enzymes. The caspases can also activate other degradative enzymes such as DNases, which begin to cleave the DNA in the nucleus.
Apoptotic cells display distinctive morphology during the apoptotic process. Typically, the cell begins to shrink following the cleavage of lamins and actin filaments in the cytoskeleton. The breakdown of chromatin in the nucleus often leads to nuclear condensation and in many cases the nuclei of apoptotic cells take on a "horse-shoe" like appearance. Cells continue to shrink, packaging themselves into a form that allows for their removal by macrophages. There are a number of mechanisms through which apoptosis can be induced in cells. The sensitivity of cells to any of these stimuli can vary depending on a number of factors such as the expression of pro- and anti-apoptotic proteins (eg. the Bcl-2 proteins or the Inhibitor of Apoptosis Proteins), the severity of the stimulus and the stage of the cell cycle. The Bcl-2 family of proteins plays a central role in the regulation of apoptotic cell death induced by a wide variety of stimuli. Some proteins within this family, including Bcl-2 and Bcl-xL, inhibit programmed cell death, and others, such as Bax and Bak, can promote apoptosis 6, 7.
Functions
For development, Apoptosis is as needed for proper development as mitosis is. Examples: The resorption of the tadpole tail at the time of its metamorphosis into a frog occurs by apoptosis.
Integrity of the organism, Apoptosis is needed to destroy cells that represent a threat to the integrity of the organism. Examples: Cells infected with viruses8.
Cells of the immune system, as cell-mediated immune responses wane, the effector cells must be removed to prevent them from attacking body constituents. CTLs induce apoptosis in each other and even in themselves 9.
Cells with DNA damage, damage to its genome can cause a cell to disrupt proper embryonic development leading to birth defects to become cancerous.
References
1. Kerr JF (1965). A histochemical study of hypertrophy and ischaemic injury of rat liver with special reference to changes in lysosomes. Journal of Pathology and Bacteriology, 90(90):419-435.
2. Kerr JF, Wyllie AH, Currie AR (1972). Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer., 26(4):239-257.
3. O'Rourke MG, Ellem KA (2000). John Kerr and apoptosis. Med. J. Aust., 173(11-12): 616-617.
4. Franklin MC, Kadkhodayan S, Ackerly H, Alexandru D, Distefano MD, Elliott LO, Flygare JA, Mausisa G, Okawa DC, Ong D, Vucic D, Deshayes K, Fairbrother WJ (2003). Structure and function analysis of peptide antagonists of melanoma inhibitor of apoptosis (ML-IAP). Biochemistry, 42(27):8223-8231.
5. Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW (1997). Structure of bcl-xl-bak peptide complex: recognition between regulators of apoptosis. Science, 275(5302):983-986.
6. Hanada M, Aimé-Sempé C, Sato T, Reed JC (1995). Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J. Biol. Chem., 270(20):11962-11969.
7. Cheng EHY, Levine B, Boise LH, Thompson CB, Hardwic JM (1996). Bax-independent inhibition of apoptosis by Bcl-xL.Nature, 379:554-556.
8. Alimonti JB, Ball TB, Fowke KR (2003). Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J Gen Virology., 84(84): 1649-1661.
9. Werlen G, Hausmann B, Naeher D, Palmer E (2003). Signaling life and death in the thymus: timing is everything. Science. 299(5614):1859-1863.
Definition
Caspases are a family of aspartate specific cysteine proteases that play an important role in apoptosis, necrosis and inflammation1.
Discovery
Caspases were first identified in the nematode C. elegans. It was found that the gene ced-3 was required for cell death during C.elegans development2. In 1993, the protein encoded by the ced-3 gene was identified as a cysteine protease and it was found that it had similar properties to the mammalian interleukin-1-beta converting enzyme (ICE) (now known as caspase 1) which at the time was the only known caspase3. Other mammalian caspases were subsequently identified.
Classification
There are three types of apoptotic caspases: initiator, effector and inflammatory caspases. Initiator caspases (e.g. CASP2, CASP8, CASP9 and CASP10) cleave inactive pro-forms of effector caspases, thereby activating them4. Effector caspases (e.g. CASP3, CASP6 and CASP7) in turn cleave other protein substrates within the cell, to trigger the apoptotic process4. Inflammatory caspases are involved in immune response (e.g. CASP1, CASP4, CASP5, CASP11, CASP12 and CASP13). Caspase inhibitors regulate the initiation of this cascade4.
Structural Characteristics
Caspases are synthesized as inactive zymogens or procaspases. Activation of caspases occurs by cleavage of the prodomain in the procaspases5. The caspase catalytic domain is composed of a twisted, mostly parallel ß-sheet sandwiched between two layers of a-helices. Also they contain an active cysteine residue in their catalytic domain5. In addition to the catalytic domain, both inflammatory and initiator caspases carry at their N-termini, one or two copies of CARD or DED modules, which are critical for their activation in vivo. These modules are mainly composed of six antiparallel a-helices, with helices a1–a5 building an a-helical Greek key5. The general structure of a caspase inhibitor is [tetrapeptide]-CO-CH2-X, that binds to the Cys285 in the active site of caspases5.
Mode of action
Caspases cleave the substrate after an Asp residue6. There are several hundred substrates for caspases. Initially activation of initiator caspases occurs as a result of an extrinsic or intrinsic death signal6. Activated initiator caspases cleave effector caspases that in turn cleave the substrate at an Asp residue6. For example, caspase-8 cleaves the pro-apoptotic protein Bid that gets activated and translocates into the mitochondria where it activates other pro-apoptotic proteins, Bax and Bak thus amplifying the death signal6.
Functions
Caspases such as caspase-1 are involved in the activation of pro-inflammatory cytokines such as Interleukin 1 and interleukin 185,6. Caspases play an important role in apoptosis. One of the hallmark feature of apoptotic cell death is genomic disassembly and proteolysis5,6. By cleaving their substartes, caspases inactivate cell cycle progression and DNA repair processes. They also activate several pro-apoptotic proteins5,6. In some cases Caspases’ role in aberrant processing events has shown their involvement in neurodegenerative disorders such as Huntington disease and Alzheimer’s disease6. Some of the final targets of caspases include: nuclear lamins, ICAD/DFF45 (inhibitor of caspase activated DNase or DNA fragmentation factor 45), PARP (poly-ADP ribose polymerase) and PAK2 (P 21-activated kinase 2)6. Caspases are also implicated in embryonic development and T and B cell differentiation7.
References
1. Book: Cells by Benjamin L, Lynne C, Vishwanath RL, George P (207), 536-540.
2. Ellis HM, Horvitz HR (1986). Genetic control of programmed cell death in the nematode C. elegans. Cell, 44(6), 817-29.
3. Yuan J, Shaham S, Ledoux S, Ellis HM and Horvitz HR (1993). The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell 75: 641–652.
4. Salvesen GS, Riedl SJ (2008). Caspase mechanisms. Adv Exp Med Biol., 615, 13-23.
5. Prior PF and Salvesen GS (2004). The protein structures that shape caspase activity, specificity,activation and inhibition. Biochem. J., 384, 201–232.
6. Nicholson DW (1999). Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death and Differentiation, 6, 1028 ± 1042.
7. Maelfait J, Beyaert R (2008). Non-apoptotic functions of caspase-8. Biochem Pharmacol., 76(11), 1365-73.
Caspase酶对应的底物,Caspases(半胱氨酸天冬氨酸蛋白酶,半胱氨酸依赖性天冬氨酸定向蛋白酶)是一类蛋白酶家族,其功能与凋亡(程序性细胞死亡),坏死和发烧(炎症)的过程密切相关。
什么是胱天蛋白酶?
胱天蛋白酶(Caspases)是含半胱氨酸的天冬氨酸蛋白水解酶,它们是为细胞凋亡的主要介质。多种受体,例如TNF-α 受体,FasL受体,TLR和死亡受体,以及Bcl-2和凋亡抑制剂(IAP)蛋白家族参与并调节该caspase依赖性凋亡途径。一旦Caspase受到上游信号(外部或内在)刺激被激活,即会参与执行下游蛋白底物的水解作用,并触发一系列事件,导致细胞分解,死亡,吞噬作用和细胞碎片的清除。
人Caspases酶
人的Caspases家族基于序列相似性和生物学功能等共性主要可分为三大类:第一类由具有长胱天蛋白酶募集结构域的“炎症”胱天蛋白酶组成,他们对P4位上的较大的芳香族或疏水性残基具有亲和力。第二类由具有短的前体结构域的“细胞凋亡效应”胱天蛋白酶组成,而第三类由具有长的前提结构域的Pap位置具有亮氨酸或缬氨酸底物亲和力的“凋亡引发剂”胱天蛋白酶组成(表1)。
表1. 人胱天蛋白酶的功能分类:
| 细胞死亡途径 | 半胱天冬酶类型 | 酵素 | 物种 |
| 细胞凋亡 | 启动器 | Caspases 2 | 人与鼠 |
| 细胞凋亡 | 启动器 | Caspases 8 | 人与鼠 |
| 细胞凋亡 | 启动器 | Caspases 9 | 人与鼠 |
| 细胞凋亡 | 启动器 | Caspases 10 | 人的 |
| 细胞凋亡 | 效应器 | Caspases 3 | 人与鼠 |
| 细胞凋亡 | 效应器 | Caspases 6 | 人与鼠 |
| 细胞凋亡 | 效应器 | Caspases 6 | 人与鼠 |
| 细胞焦亡 | 炎性的 | Caspases 1 | 人与鼠 |
| 细胞焦亡 | 炎性的 | Caspases 4 | 人的 |
| 细胞焦亡 | 炎性的 | Caspases 5 | 人的 |
启动器Caspase和效应器Caspase酶
根据其在凋亡胱天蛋白酶途径中的作用,胱天蛋白酶可分为两类:启动器和效应器Caspase酶。启动器和效应器Caspas酶都具有由小亚基和大亚基组成的催化位点,Caspase酶的识别位
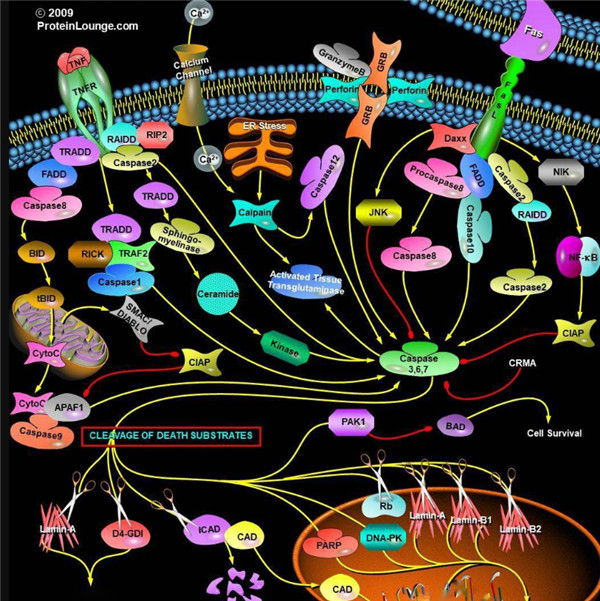
凋亡启动器Caspase酶,例如caspase-2,-8,-9和-10可以启动caspase激活级联反应。Caspase-8对于形成死亡诱导信号复合物(DISC)是必不可少的,并且在激活后,Caspase-8激活下游效应子Caspase(例如Caspase 3)并介导线粒体中细胞色素c的释放。Caspase-8已被证明对IETD肽序列具有相对较高的底物选择性。凋亡效应胱天蛋白酶例如Caspase-3,-6和-7虽然不负责启动级联途径,但是当被激活时,它们在级联的中间和后续步骤中起着不可或缺的作用。Caspase-3(CPP32 / apopain)是关键效应器,因为它放大了来自启动器Caspase的信号,使用对Caspase-3有选择性的DEVD肽序列对活化的Caspase-3进行检测,可以检测Caspase-3的活性。
Caspase酶底物和抑制剂
Caspase底物和抑制剂由两个关键成分组成:Caspase识别序列和信号产生或蛋白酶抑制基序。不同Caspase识别序列不同,一般由三个或四个氨基酸组成(表2)。Caspase酶识别序列的N端通常有乙酰基(Ac)或碳苯甲氧基(Z)基团修饰,以增强膜的通透性。对应的Caspase识别特定的肽序列为其酶促反应切割位点,释放产生信号或抑制信号的基序。Caspase的显色和荧光底物均以相似的方式起作用,其中底物的信号或颜色强度与蛋白水解活性成正比。
表2. Caspase的底物及其序列
| 多肽 | 氨基酸序列 | 对应的Caspase的种类 |
| IETD | Ile-Glu-Thr-Asp | Caspase 8,颗粒酶B |
| DEVD | Asp-Glu-Val-Asp | Caspase 3、6、7、8或10 |
| LEHD | Leu-Glu-His-Asp | Caspase 9 |
| VAD | Val-Ala-Asp | Caspase 1、2、3、6、8、9或10 |
Caspase酶的显色底物
Caspase的显色底物是有Caspase识别序列及生色基团组成,常见的生色团有pNA(对硝基苯胺或4-硝基苯胺),可使用酶标仪或分光光度计在405 nm处进行光密度检测。
表3. Caspase的显色底物
| 底物 | Caspase | 吸收(nm) | 颜色 |
| Ac-DEVD-pNA * CAS 189950-66-1 * | 半胱天冬酶3 | 405 nm | 黄色 |
| Z-DEVD-pNA | 半胱天冬酶3 | 405 nm | 黄色 |
| Z-IETD-pNA * CAS 219138-21-3 * | 半胱天冬酶8,颗粒酶B | 405 nm | 黄色 |
Caspase的荧光底物
Caspase的荧光底物的结构包含与半胱天冬酶识别相关的荧光团,例如7-氨基-4-甲基香豆素(AMC),7-氨基-4-三氟甲基香豆素(AFC), Rhodamine 110(R110)或ProRed™620。R110的Caspase底物比基于香豆素的Caspase底物(例如AMC和AFC)更敏感,但由于两步裂解过程,其动态范围更窄。 建议将R110标记的Caspase底物用于终点法测定,而将AMC和AFC标记的 Caspase底物用于动力学测定。
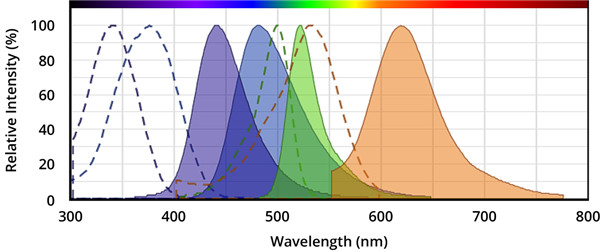
图.从左到右,分别是AMC(7-氨基-4-甲基香豆素),AFC(7-氨基-4-三氟甲基香豆素),Rhodamine 110(R110)和ProRed™620的激发和发射光谱。
表4.荧光半胱天冬酶底物。
| 底物名称 | 对应的Caspase | Ex(nm) | Em(nm) | ε¹ | Φ² |
| Ac-DEVD-AFC * CAS 201608-14-2 * | 半胱天冬酶3、7 | 376 | 482 | 17000 | 0.53 |
| Ac-DEVD-AMC * CAS 169332-61-0 * | 半胱天冬酶3、7 | 341 | 441 | 19000 | N / D |
| Z-DEVD-AFC | 半胱天冬酶3、7 | 376 | 482 | 17000 | 0.53 |
| Z-DEVD-AMC * CAS 1135416-11-3 * | 半胱天冬酶3、7 | 341 | 441 | 19000 | N / D |
| Z-DEVD-ProRed™620 | 半胱天冬酶3、7 | 532 | 619 | N / D | N / D |
| (Z-DEVD)2 -R110 * CAS 223538-61-2 * | 半胱天冬酶3、7 | 500 | 522 | 80000 | N / D |
| Z-DEVD-ProRed™620 | 半胱天冬酶3、7 | 532 | 619 | N / D | N / D |
| Ac-IETD-AFC * CAS 211990-57-7 * | 半胱天冬酶8,颗粒酶B | 376 | 482 | 17000 | 0.53 |
| Z-IETD-AFC * CAS 219138-02-0 * | 半胱天冬酶8,颗粒酶B | 376 | 482 | 17000 | 0.53 |
注意:
1.ε=在其最大吸收波长处的摩尔消光系数(单位= cm -1M -1)。
2.Φ=水性缓冲液(pH 7.2)中的荧光量子产率。
Caspase抑制剂
Caspase抑制剂能与Caspase的活性位点结合并形成可逆或不可逆的连接,通常,Caspase抑制剂的结构由Caspase识别序列,诸如醛(-CHO)或氟甲基酮(-FMK)的官能团组成。具有醛官能团的胱天蛋白酶抑制剂是可逆的,而具有FMK的抑制剂是不可逆的。半胱天冬酶底物和抑制剂都具有较小的细胞毒性作用,因此,它们是研究半胱天冬酶活性的有用工具。
表5. 可逆和不可逆的Caspase酶抑制剂
| 抑制剂 | Caspase的种类 | 是否可逆 | Ex(nm) | Em(nm) |
| Ac-DEVD-CHO * CAS 169332-60-9 * | 半胱天冬酶3、7 | 可逆的 | -- | -- |
| Ac-IETD-CHO * CAS 191338-86-0 * | 半胱天冬酶8 | 可逆的 | -- | -- |
| mFluor™450-VAD-FMK | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 406 | 445 |
| mFluor™510-VAD-FMK | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 412 | 505 |
| FITC-C6-DEVD-FMK | 半胱天冬酶3、7 | 不可逆的 | 491 | 516 |
| FITC-C6-DEVD-FMK | 半胱天冬酶3、7 | 不可逆的 | 491 | 516 |
| FITC-C6-LEHD-FMK | 半胱天冬酶9 | 不可逆的 | 491 | 516 |
| FITC-C6-LEHD-FMK | 半胱天冬酶9 | 不可逆的 | 491 | 516 |
| FAM-VAD-FMK | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 493 | 517 |
| SRB-VAD-FMK [磺胺丁胺B-VAD-FMK] | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 559 | 577 |
定义
酶是用于生化反应的非常有效的催化剂。它们通过提供较低活化能的替代反应途径来加快反应速度。酶作用于底物并产生产物。一些物质降低或什至停止酶的催化活性被称为抑制剂。
发现
1965年,Umezawa H分析了微生物产生的酶抑制剂,并分离出了抑制亮肽素和抗痛药的胰蛋白酶和木瓜蛋白酶,乳糜蛋白酶抑制的胰凝乳蛋白酶,胃蛋白酶抑制素抑制胃蛋白酶,泛磷酰胺抑制唾液酸酶,乌藤酮抑制酪氨酸羟化酶,多巴汀抑制多巴胺3-羟硫基嘧啶和多巴胺3-羟色胺酶酪氨酸羟化酶和多巴胺J3-羟化酶。最近,一种替代方法已应用于预测新的抑制剂:合理的药物设计使用酶活性位点的三维结构来预测哪些分子可能是抑制剂1。已经开发了用于识别酶抑制剂的基于计算机的方法,例如分子力学和分子对接。
结构特征
已经确定了许多抑制剂的晶体结构。已经确定了三种与凝血酶复合的高效且选择性的低分子量刚性肽醛醛抑制剂的晶体结构。这三种抑制剂全部在P3位置具有一个新的内酰胺部分,而对胰蛋白酶选择性最高的两种抑制剂在P1位置具有一个与S1特异性位点结合的胍基哌啶基。凝血酶的抑制动力学从慢到快变化,而对于胰蛋白酶,抑制的动力学在所有情况下都快。根据两步机理2中稳定过渡态络合物的缓慢形成来检验动力学。
埃米尔•菲舍尔(Emil Fischer)在1894年提出,酶和底物都具有特定的互补几何形状,彼此恰好契合。这称为“锁和钥匙”模型3。丹尼尔·科什兰(Daniel Koshland)提出了诱导拟合模型,其中底物和酶是相当灵活的结构,当底物与酶4相互作用时,活性位点通过与底物的相互作用不断重塑。
在众多生物活性肽的成熟过程中,需要由其谷氨酰胺(或谷氨酰胺)前体形成N末端焦谷氨酸(pGlu)。游离形式并与底物和三种咪唑衍生抑制剂结合的人QC的结构揭示了类似于两个锌外肽酶的α/β支架,但有多个插入和缺失,特别是在活性位点区域。几种活性位点突变酶的结构分析为针对QC相关疾病5的抑制剂的合理设计提供了结构基础。
作用方式
酶是催化化学反应的蛋白质。酶与底物相互作用并将其转化为产物。抑制剂的结合可以阻止底物进入酶的活性位点和/或阻止酶催化其反应。抑制剂的种类繁多,包括:非特异性,不可逆,可逆-竞争性和非竞争性。可逆抑制剂 以非共价相互作用(例如疏水相互作用,氢键和离子键)与酶结合。非特异性抑制方法包括最终使酶的蛋白质部分变性并因此不可逆的任何物理或化学变化。特定抑制剂 对单一酶发挥作用。大多数毒药通过特异性抑制酶发挥作用。竞争性抑制剂是任何与底物的化学结构和分子几何结构非常相似的化合物。抑制剂可以在活性位点与酶相互作用,但是没有反应发生。非竞争性抑制剂是与酶相互作用但通常不在活性位点相互作用的物质。非竞争性抑制剂的净作用是改变酶的形状,从而改变活性位点,从而使底物不再能与酶相互作用而产生反应。非竞争性抑制剂通常是可逆的。不可逆抑制剂与酶形成牢固的共价键。这些抑制剂可以在活性位点附近或附近起作用。
功能
工业应用中, 酶在商业上被广泛使用,例如在洗涤剂,食品和酿造工业中。蛋白酶用于“生物”洗衣粉中,以加速蛋白质在诸如血液和鸡蛋等污渍中的分解。商业上使用酶的问题包括:它们是水溶性的,这使得它们难以回收,并且一些产物可以抑制酶的活性(反馈抑制)。
药物分子,许多药物分子都是酶抑制剂,药用酶抑制剂通常以其特异性和效力为特征。高度的特异性和效力表明该药物具有较少的副作用和较低的毒性。酶抑制剂在自然界中发现,并且也作为药理学和生物化学的一部分进行设计和生产6。
天然毒物 通常是酶抑制剂,已进化为保护植物或动物免受天敌的侵害。这些天然毒素包括一些已知最剧毒的化合物。
神经气体( 例如二异丙基氟磷酸酯(DFP))通过与丝氨酸的羟基反应生成酯,从而抑制了乙酰胆碱酯酶的活性位点。
参考
1、Scapin G (2006). Structural biology and drug discovery. Curr. Pharm. Des., 12(17):2087–2097.
2、Krishnan R, Zhang E, Hakansson K, Arni RK, Tulinsky A, Lim-Wilby MS, Levy OE, Semple JE, Brunck TK (1998). Highly selective mechanism-based thrombin inhibitors: structures of thrombin and trypsin inhibited with rigid peptidyl aldehydes. Biochemistry, 37 (35):12094-12103.
3、Fischer E (1894). Einfluss der configuration auf die wirkung der enzyme. Ber. Dt. Chem. Ges., 27:2985–2993.
4、Koshland DE (1958). Application of a theory of enzyme specificity to protein synthesis. PNAS., 44 (2):98–104.
5、Huang KF, Liu YL, Cheng WJ, Ko TP, Wang AH (2005). Crystal structures of human glutaminyl cyclase, an enzyme responsible for protein N-terminal pyroglutamate formation. PNAS., 102(37):13117-13122.
6、Holmes CF, Maynes JT, Perreault KR, Dawson JF, James MN (2002). Molecular enzymology underlying regulation of protein phosphatase-1 by natural toxins. Curr Med Chem., 9(22):1981-1989.
Definition
Enzymes are very efficient catalysts for biochemical reactions. They speed up reactions by providing an alternative reaction pathway of lower activation energy. Enzyme acts on substrate and gives rise to a product. Some substances reduce or even stop the catalytic activities of enzymes are called inhibitors.
Discovery
In 1965, Umezawa H analysed enzyme inhibitors produced by microorganisms and isolated leupeptin and antipain inhibiting trypsin and papain, chymostatin inhibiting chymotrypsin, pepstatin inhibiting pepsin, panosialin inhibiting sialidases, oudenone inhibiting tyrosine hydroxylase, dopastin inhibiting dopamine 3-hydroxylase, aquayamycin and chrothiomycin inhibiting tyrosine hydroxylase and dopamine J3-hydroxylase . Recently, an alternative approach has been applied to predict new inhibitors: rational drug design uses the three-dimensional structure of an enzyme's active site to predict which molecules might be inhibitors 1. Computer-based methods for identifying inhibitor for an enzyme have been developed, such as molecular mechanics and molecular docking.
Structural Characteristics
The crystal structures of many inhibitors have been determined. The crystal structures of three highly potent and selective low-molecular weight rigid peptidyl aldehyde inhibitors complexed with thrombin have been determined. All the three inhibitors have a novel lactam moiety at the P3 position, while the two with greatest trypsin selectivity have a guanidinopiperidyl group at the P1 position that binds in the S1 specificity site. The kinetics of inhibition vary from slow to fast with thrombin and are fast in all cases with trypsin. The kinetics are examined in terms of the slow formation of a stable transition-state complex in a two-step mechanism 2.
Emil Fischer in 1894 suggested that both the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another.This is known as "the lock and key" model 3. Daniel Koshland suggested induced fit model where substrate and enzymes are rather flexible structures, the active site is continually reshaped by interactions with the substrate as the substrate interacts with the enzyme 4.
N-terminal pyroglutamate (pGlu) formation from its glutaminyl (or glutamyl) precursor is required in the maturation of numerous bioactive peptides. The structure of human QC in free form and bound to a substrate and three imidazole-derived inhibitors reveals an alpha/beta scaffold akin to that of two-zinc exopeptidases but with several insertions and deletions, particularly in the active-site region. The structural analyses of several active-site-mutant enzymes provide a structural basis for the rational design of inhibitors against QC-associated disorders 5.
Mode of Action
Enzymes are proteins that catalyze chemical reactions. Enzymes interact with substrate and convert them into products. Inhibitor binding can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. There are a variety of types of inhibitors including: nonspecific, irreversible, reversible - competitive and noncompetitive. Reversible inhibitors bind to enzymes with non-covalent interactions like hydrophobic interactions, hydrogen bonds, and ionic bonds. Non-specific methods of inhibition include any physical or chemical changes which ultimately denature the protein portion of the enzyme and are therefore irreversible. Specific Inhibitors exert their effects upon a single enzyme. Most poisons work by specific inhibition of enzymes. A competitive inhibitor is any compound which closely resembles the chemical structure and molecular geometry of the substrate. The inhibitor may interact with the enzyme at the active site, but no reaction takes place. A noncompetitive inhibitor is a substance that interacts with the enzyme, but usually not at the active site. The net effect of a non competitive inhibitor is to change the shape of the enzyme and thus the active site, so that the substrate can no longer interact with the enzyme to give a reaction. Non competitive inhibitors are usually reversible. Irreversible Inhibitors form strong covalent bonds with an enzyme. These inhibitors may act at, near, or remote from the active site .
Functions
Industrial application, enzymes are widely used commercially, for example in the detergent, food and brewing industries. Protease enzymes are used in 'biological' washing powders to speed up the breakdown of proteins in stains like blood and egg. Problems using enzymes commercially include: they are water soluble which makes them hard to recover and some products can inhibit the enzyme activity (feedback inhibition) .
Drug molecules, many drug molecules are enzyme inhibitors and a medicinal enzyme inhibitor is usually characterized by its specificity and its potency. A high specificity and potency suggests that a drug will have fewer side effects and less toxic. Enzyme inhibitors are found in nature and are also designed and produced as part of pharmacology and biochemistry 6.
Natural poisons are often enzyme inhibitors that have evolved to defend a plant or animal against predators. These natural toxins include some of the most poisonous compounds known.
Nerve gases such as diisopropylfluorophosphate (DFP) inhibit the active site of acetylcholine esterase by reacting with the hydroxyl group of serine to make an ester.
References
Scapin G (2006). Structural biology and drug discovery. Curr. Pharm. Des., 12(17):2087–2097.
Krishnan R, Zhang E, Hakansson K, Arni RK, Tulinsky A, Lim-Wilby MS, Levy OE, Semple JE, Brunck TK (1998). Highly selective mechanism-based thrombin inhibitors: structures of thrombin and trypsin inhibited with rigid peptidyl aldehydes. Biochemistry, 37 (35):12094-12103.
Fischer E (1894). Einfluss der configuration auf die wirkung der enzyme. Ber. Dt. Chem. Ges., 27:2985–2993.
Koshland DE (1958). Application of a theory of enzyme specificity to protein synthesis. PNAS., 44 (2):98–104.
Huang KF, Liu YL, Cheng WJ, Ko TP, Wang AH (2005). Crystal structures of human glutaminyl cyclase, an enzyme responsible for protein N-terminal pyroglutamate formation. PNAS., 102(37):13117-13122.
Holmes CF, Maynes JT, Perreault KR, Dawson JF, James MN (2002). Molecular enzymology underlying regulation of protein phosphatase-1 by natural toxins. Curr Med Chem., 9(22):1981-1989.
| DOI | 名称 | |
|---|---|---|
| 10.1080/10428190701385181 | The tyrosine kinase inhibitor AMN107 (Nilotinib) exhibits off-target effects in lymphoblastic cell lines | 下载 |
| 10.1016/j.jdermsci.2007.06.008 | Flavonolignans from Silybum marianum moderate UVA-induced oxidative damage to HaCaT keratinocytes | 下载 |
| 10.1002/cbdv.200800354 | Antiproliferative and proapoptotic activities of a new class of pyrazole derivatives in HL-60 cells | 下载 |





