400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研
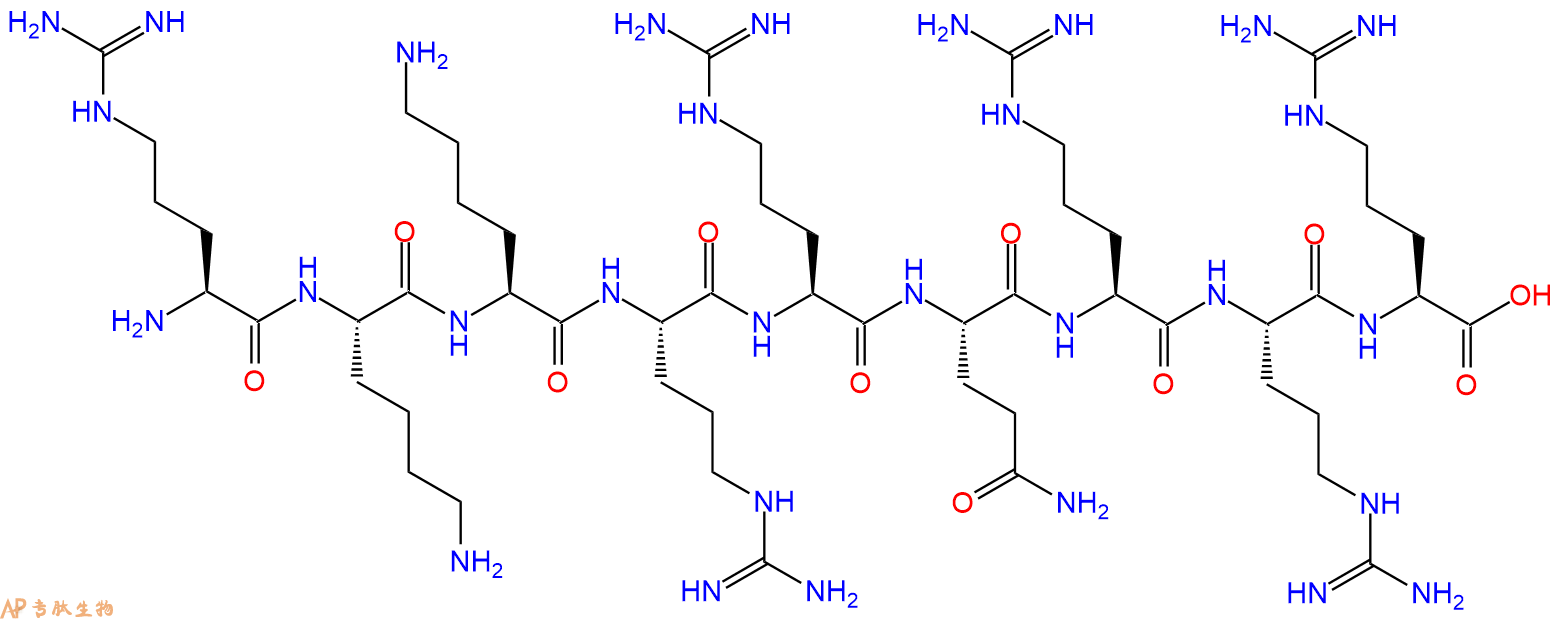
是HIV-1 tat蛋白转导域(PTD)中的九个氨基酸基序,足以通过细胞膜转导异源蛋白。
编号:168983
CAS号:123251-89-8
单字母:H2N-RKKRRQRRR-OH
| 参考文献(References): | J.Park et al., J. Gen. Virol., 83, 1173 (2002) |
RKKRRQRRR是HIV-1 tat蛋白转导结构域(PTD)内的一个九个氨基酸的基序,足以通过质膜转导异源蛋白。
RKKRRQRRR, a nine amino acid motif within the HIV-1 tat protein transduction domain (PTD), suffices for the transduction of heterologous proteins through the plasma membrane.
Definition
Human immunodeficiency virus (HIV) is a lentivirus that causes acquired immunodeficiency syndrome (AIDS), a condition in humans in which the immune system begins to fail, leading to life-threatening opportunistic infections. Over 5000 HIV-related peptides have been synthesized, that inhibit different stages of viral life cycle.
Discovery
In 1983, two separate researchers Robert Gallo and Luc Montagnier independently declared that a novel retrovirus infecting AIDS patients. Several HIV related peptides including peptides (15-mers or 20-mers) of HIV glycoprotein 160 (gp160), gp120W16D, MN envelope (env) consensus B tat, consensus B VIF, HXB2 gag, SIVmac239, SIVmac239env, SIVmac239 gag have been used to study HIV life cycle. C34 peptide of Gp41 HIV Fragment is known as HR2, belongs to the helical region of gp41 of HIV, C-terminal heptad repeat 2 (HR2) defined as C helix or C peptide. It is known that HIV-1 enters cells by membrane fusion, C34 gp41 peptide is a potent inhibitors of HIV-1 fusion 1,2. The 86 amino acid trans-activator (Tat) protein of human immunodeficiency virus type 1 (HIV-1) is an RNA-binding transcriptional regulator. HIV-1 Tat proteins (wild type and Thr40Lys mutant) and the HIV-1 Tat peptide fragments Tat(32–48) and Tat(32–72) were chemically synthesized and used for HIV studies 3.
HIV (gp120) fragment (254-274), this fragment with sequence homology to a domain of the external envelope glycoprotein (gp120) of the human immunodeficiency virus (HIV) is important for HIV infectivity and antibody neutralization 4. HIV (gp120) fragment (421-438), derived from the CD4 attachment region of HIV gp120, inhibited the syncytial formation in vitro 5. HIV-1 gag protein p17 (76-84), HLA-A*0201-restricted immunodominant CD8 epitope of the HIV gag protein used for the characterization of CD8+ -T cells of HIV-positiv patients 6. HIV-1 rev protein (34-50), this arginine-rich fragment interacts specifically with RNA. It has been shown that rev protein and rev protein (34-50) bind IIB RNA with a similar dissociation constant of approx. 10 nM 7.
Structural Characteristics
The HIV type-1 belongs to the family Retroviridae and consists of two basic components: a core of ribonucleic acid (RNA), called the genome, and a protein component that surrounds the genome, called a capsid. The single-stranded RNA is tightly bound to nucleocapsid proteins and enzymes needed for the morphogenesis of the virion such as reverse transcriptase, proteases, ribonuclease and integrase. A matrix composed of the viral protein that surrounds the capsid. Viral envelope is composed of two layers of fatty molecules taken from the membrane of a human cell during budding process. There are 70 copies of a complex HIV protein that protrudes through the surface of the virus particle, known as Env, consists of a cap, glycoprotein (gp) 120, and a stem, gp41 molecules. This glycoprotein complex is important for fusion of virus to host cell. Both these surface proteins are important targets for treatments or HIV vaccines 8.
Mode of Action
HIV binds to a CD4 receptor and one of two co-receptors on the surface of a CD4+ T- lymphocyte. After fusion, the virus releases RNA, its genetic material, into the host cell. An HIV enzyme called reverse transcriptase converts the single- stranded HIV RNA to double-stranded HIV DNA. The newly formed HIV DNA enters the host cell's nucleus. The integrated HIV DNA is called provirus. The provirus may remain inactive for several years, producing few or no new copies of HIV. When the host cell receives a signal to become active, the provirus uses a host enzyme called RNA polymerase to create copies of the HIV genomic material, as well as shorter strands of RNA called messenger RNA (mRNA). The mRNA is used as a blueprint to make long chains of HIV proteins. An HIV enzyme called protease cuts the long chains of HIV proteins into smaller individual proteins. As the smaller HIV proteins come together with copies of HIV's RNA genetic material, a new virus particle is assembled. The newly assembled virus buds out from the host cell. During budding, the new virus acquires part of the cell's outer envelope. This envelope is embedded with viral glycoproteins which are necessary for host cell recognition.
Functions
CD8 cytotoxic, HIV-1 specific CD8 cytotoxic T lymphocyte (CTL) responses play a critical role in controlling HIV-1 replication. TCR avidity correlates with CTL function, and CTLs expressing TCRs with high avidity for their cognate MHC-viral peptide complex play an important in vivo role in neutralizing virus infections, terminating virus infection and delaying systemic AIDS virus dissemination from the mucosal inoculation site.
HIV-1 envelope transmembrane protein that contain highly positively charged amphipathic helices (designated LLP) in have both cytolytic and calmodulin (CaM) binding/inhibitory properties that contribute to cytopathogenesis during a viral infection.
HIV-1 vif, The human immunodeficiency virus type 1 (HIV-1) auxiliary gene vif is essential for virus propagation in peripheral blood lymphocytes, macrophages, and in some T-cell lines. (i) Vif protein binds HIV-1 PR (protease), but not covalently linked tethered PR-PR; (ii) the four amino acids residing at the N terminus of HIV-1 PR are essential for Vif/PR interaction; (iii) synthetic peptide derived from the N terminus of HIV-1 PR inhibits Vif/PR binding; and (iv) this peptide inhibits the propagation of HIV-1 in restrictive cells 9.
References
1. Bianchi E, Finotto M, Ingallinella P, Hrin R, Carella AV, Hou XS, Schleif WA, Miller MD, Geleziunas R, Pessi A (2005). Covalent stabilization of coiled coils of the HIV gp41 N region yields extremely potent and broad inhibitors of viral infection. PNAS., 102(36):12903-12908
2. de Rosny E, Vassell R, Jiang S, Kunert R, Weiss CD (2004). Binding of the 2F5 monoclonal antibody to native and fusion-intermediate forms of human immunodeficiency virus type 1 gp41: implications for fusion-inducing conformational changes. J. Virol., 78(5):2627-2631.
3. Klostermeier D, Bayer P, Kraft M, Frank RW, Rösch P (1997). Spectroscopic investigations of HIV-1 trans-activator and related peptides in aqueous solutions. Biophysical Chemistry, 63(2):87-96.
4. Ho DD, Kaplan JC, Rackauskas IE, Gurney ME (1988). Second conserved domain of gp120 is important for HIV infectivity and antibody neutralization. Science, 239(4843):1021-1023.
5. Morrow WJ, Williams WM, Whalley AS, Ryskamp T, Newman R, Kang CY, Chamat S, Köhler H, Kieber-Emmons T (1992). Synthetic peptides from a conserved region of gp120 induce broadly reactive anti-HIV responses. Immunology, 75(4):557-564.
6. Wilkinson J, Cope A, Gill J, Bourboulia D, Hayes P, Imami N, Kubo T, Marcelin A, Calvez V, Weiss R, Gazzard B, Boshoff C, Gotch F (2002). Identification of Kaposi's sarcoma-associated herpesvirus (KSHV)-specific cytotoxic T-lymphocyte epitopes and evaluation of reconstitution of KSHV-specific responses in human immunodeficiency virus type 1-Infected patients receiving highly active antiretroviral therapy. J. Virol., 76(6):2634-2640.
7. Kjems J, Calnan BJ, Frankel AD, Sharp PA (1992). Specific binding of a basic peptide from HIV-1 Rev. EMBO J., 11(3):1119-29.
8. Chan DC, Fass D, Berger JM, Kim PS (1997). Core structure of gp41 from the HIV envlope glycoprotein . Cell, 89:263–73.
9. Hutoran M, Britan E, Baraz L, Blumenzweig I, Steinitz M, Kotler M (2004). Abrogation of Vif function by peptide derived from the N-terminal region of the human immunodeficiency virus type 1 (HIV-1) protease. Virology, 330(1):261-270.
细胞穿膜肽-说明
穿透细胞膜进入细胞内是许多作用靶点在细胞内的生物大分子发挥作用的先决条件,然而生物膜的生物屏障作用阻止了许多高分子物质进入细胞内,从而很大程度地限制了这些物质在治疗领域的应用。因此,如何引导这些物质穿透细胞膜是一个迫切需要解决的问题,目前介导生物大分子穿透细胞膜的方法主要包括细胞穿透肽(cell penetrating peptides,CPPs)、脂质体、腺病毒、纳米颗粒、影细胞等,而CPPs是一类以非受体依赖方式,非经典内吞方式直接穿过细胞膜进入细胞的多肽,它们的长度一般不超过30个氨基酸且富含碱性氨基酸,氨基酸序列通常带正电荷。
1型人免疫缺陷病毒转录激活因子TAT(human immunodeficiency virus-1 transcription activator, HIV-1 TAT)是第一个被发现的细胞穿透肽,它凭借一种无毒的、高效的方式进入细胞。
细胞穿透肽(cell penetrating peptides,CPPs)的一个重要特点是可以携带多种不同大小和性质的生物活性物质进入细胞,包括小分子化合物、染料、多肽、多肽核酸(peptide nucleo acid, PNA)、蛋白质、质粒DNA、siRNA、200nm的脂质体、噬菌体颗粒和超顺磁性粒子等,这一性质为其成为靶向药物的良好载体提供了可能。
CPPs作为载体的优势在于低毒性和无细胞类型的限制,尽管CPPs可输送不同类型的物质进入细胞,但其实际应用多集中于寡肽、蛋白质、寡聚核苷(oligonucleotides,ONs)或类似物的细胞转运。
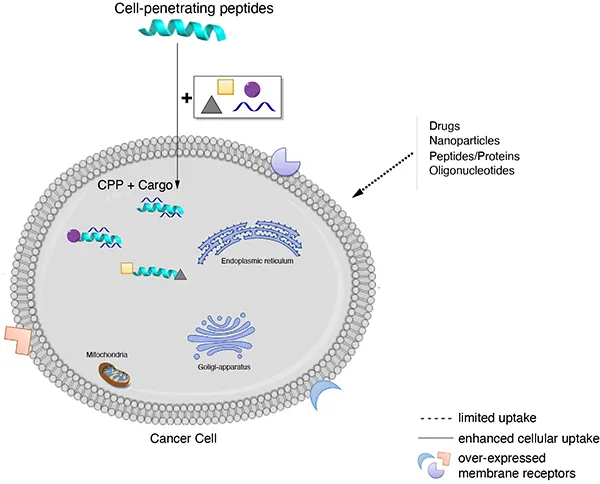
跨膜机理
不同的细胞穿透肽(CPP)跨膜机制不同,一个细胞穿透肽(CPP)的具体机制有赖于几个参数,如分子大小(携带物质)、温度、细胞类型和细胞内外的稳定性等。细胞穿透肽(CPP)进入细胞的具体机制目前还不清楚,比较流行的推测包括以下三种:
A: 倒置胶粒模型(inverted micelle model),CPPs通过细胞膜上磷脂分子的移动形成倒置胶粒结构,而进入胞浆。
B: 直接穿透,即孔隙结构模型 (pore formation model),CPPs在细胞膜上组成跨膜的孔隙结构而进入胞浆 。
C: 内吞方式进行细胞摄取。
来源: Cell-penetrating peptides and their therapeutic applications, Victoria Sebbage, BioscienceHorizons, Volume 2, Number 1, March 2009.
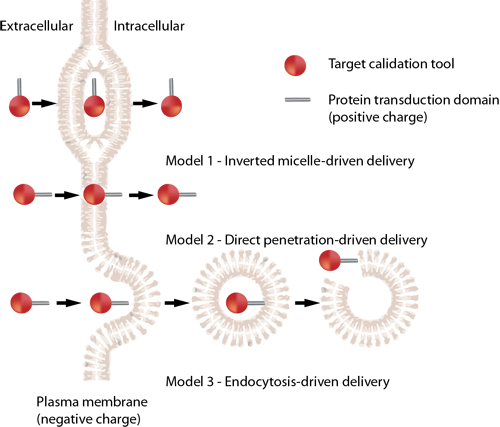
细胞穿透肽 HIV TAT
细胞穿透肽(如HIV TAT)可以以直接穿透和内吞两种方式进入细胞。HIV TAT或者简单的多聚精氨酸可被设计作为有效的药物载体,但CPP(如HIV TAT)是如何实现胞膜转运,目前仍不清楚。
简单的HIV TAT是如何促进象直接穿透和内吞作用的入胞机制的呢?来自Gerard Wong实验室的研究人员研究了在不同的条件下,HIV TAT是如何与细胞质膜、细胞骨架、特异的胞膜受体相互作用,从而诱导了多重转运途径。
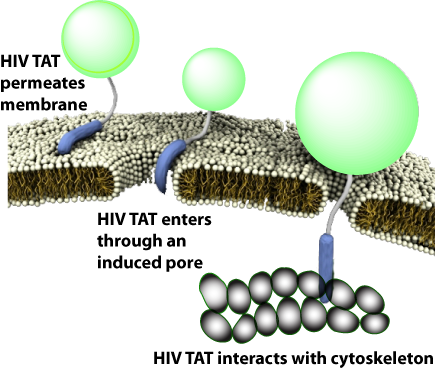
有趣的是,TAT在不同条件下可与同一序列发生多种不同的反应,因而与胞膜、细胞骨架、特异受体相互作用可产生多种转运途径。
CPP的跨膜机制与多肽序列存在很敏感的关系,如果在一个纯亲水性的CPP中增加一个疏水残基,就能彻底地改变其转运机制,例如,最简单的CPP原型-多聚精氨基(polyR),可以诱导细胞膜上形成跨膜的孔隙结构。疏水氨基酸通过插入胞膜来形成正曲率,精氨酸可同时形成正曲率和负曲率,赖氨酸只能沿一个方向形成负曲率,这就意味着在精氨酸与赖氨酸/疏水物之间存在补偿关系。
如果疏水性有助于形成负高斯曲率(Gaussian curvature),那为什么TAT肽中的疏水含量相对较低呢?其原因是CPPs都是利用尽可能少的疏水基去形成saddle-splay curvature。序列上的差异很可能只会在膜上诱导短暂的类似孔隙的跨膜结构,从而形成对CPP来说更短的孔隙寿命。由于CPP的氨基酸组成不同,TAT肽在有或无受体情况下都可以介导细胞内吞作用。
专肽生物提供各类细胞穿膜肽序列,部分由现货,例如TAT,R8,R4等,具体可咨询销售人员。
Definition
Cell permeable peptides (CPPs) are carriers with small peptide domains that can freely cross cell membranes. They are mainly used as carriers of proteins and nucleic acids into the cell1.
Discovery
The first CPP was discovered independently by two laboratories in 1988 when it was found that the trans-activating transcriptional activator (Tat) from Human Immunodeficiency Virus 1 (HIV-1) could be efficiently taken up from the surrounding media by numerous cell types in culture2.
Structural Characteristics
CPPs typically have an amino acid composition containing either a high relative abundance of positively charged, cationic amino acids such as lysine or arginine, or have sequences that contain an alternating pattern of polar/charged amino acids and non-polar, hydrophobic amino acids3. Some examples include: TAT peptide-YGRKKRRQRRR, lipid membrane translocating peptide-KKAAAVLLPVLLAAP and Antennapedia leader peptide-KKWKMRRNQFWVKVQRG.
Classification
Numerous CPPs have been identified to date and they belong to a wide variety of protein families. For example, some CPPs are amphipathic protein family members3.
Mode of action
CPPs enter the cell with their carrier by either of three mechanisms: Direct delivery that involves energy independent entry of the CPPs in to the cell4, endocytosis where the cells take up the CPPs by imbibing them with their cell membranes5 and translocation through the formation of transient structures which is yet to be understood6.
Functions
CPPs have found numerous applications in medicine as drug delivery agents in the treatment of different diseases including cancer, virus inhibitors, contrast agents for cell labeling a classical example is Green Fluorescent protein GFP, as MRI contrast agents, quantum dots7. TAT is very effective in delivering drugs in vitro and in vivo and so far a peptide that matches its efficiency has not been found7.
References
1. Wagstaff KM and David JA (2006). Protein Transduction: Cell Penetrating Peptides and Their Therapeutic Applications, Current Medicinal Chemistry, 13 (12), 1371-1387.
2. Feng S and Holland EC (1988). HIV-1 Tat trans-activation requires the loop sequence within Tar. Nature 334, 165–167.
3. Stewart KM, Horton KL, Kelley SO (2008). Cell-penetrating peptides as delivery vehicles for biology and medicine, Org Biomol Chem., 6(13), 2242-55.
4. Luo D, Saltzman WM (2000). Synthetic DNA delivery systems. Nat. Biotechnol, 18, 33-37.
5. Lundberg M., Wikstrom S and Johansson M (2003). Cell surface adherence and endocytosis of protein transduction domains, Mol. Ther., 8, 143–150.
6. Deshayes S, Gerbal-Chaloin S, Morris MC, Aldrian-Herrada G, Charnet P, Divita G (2004). On the mechanism of non-endosomial peptide-mediated cellular delivery of nucleic acids, Biochim. Biophys. Acta, 1667, 141–147.
7. Temsamani J and Vida P (2004). The use of cell-penetrating peptides for drug delivery, Drug Discovery Today, 9 (23), 1012-1019.
抗菌肽介绍一
AMPs是由相对较小的分子组成的异质基团,通常含有不到100个氨基酸。 它们最初是在20世纪60年代由Zeya和Spitznagel 在多形核白细胞溶酶体中描述的。 迄今为止,已在数据库(如数据库)中 确定和登记了2600多个AMP。 它们是由几乎所有的生物群产生的,包括细菌、真菌、植物和动物。 许多脊椎动物AMPs是由上皮表面分泌的,如 哺乳动物的气管、舌、肠粘膜或两栖动物的皮肤。 有些在中性粒细胞、单核 细胞和巨噬细胞中表达。 AMPs参与动物和植物的免疫防御系统。 构成表达或诱导它们在抵御微生物入侵者 的第一道防线中起着关键作用。
结构/分类 AMPs可以根据其氨基酸组成和结构进行分类。 可以区分两大类AMP。
第一类由线性分子组成,它们要么倾向于采用α螺旋结构,要么富含精氨酸、甘氨 酸、组氨酸、脯氨酸和色氨酸等某些氨 基酸。
第二类由含半胱氨酸的肽组成, 可分为单一或多个二硫结构。 在许多情 况下,抗菌活性需要存在二硫桥。 大多数AMPs是阳离子肽,但也有阴离子肽,如真皮素,一种富含天冬氨酸 的人肽和两栖动物的最大蛋白H5皮肤。 其他非阳离子AMPs包括神经肽前体分子的片段,如原啡肽A, 芳香二肽主要从二翅目幼虫中分离出来,或从节肢动物或茴香物种的氧结合 蛋白中提取的肽。
专肽生物可定制合成各类序列的抗菌肽,可标记FITC/FAM/TAMRA等常见荧光素。
Definition
Antimicrobial peptides (AMPs) are as widespread as bacterial inactivator molecules in the innate immune systems of insects, fungi, plants, and mammals. These peptides are also known as host defense peptides (HDPs) as they have other immuno-modulatory functions besides the direct antimicrobial actions and are even capable of killing cancerous cells 1,2.
Classification
Three broad categories of HDPs have been identified: 1) the linear peptides with helical structures, 2) the cysteine stabilized peptides with beta-sheet, and 3) a group of linear peptides rich in proline and arginine that primarily have been identified in non-mammalian species.
Structural characteristics
In mammals, cathelicidins and defensins are the two principal AMP families. Cathelicidins are peptides with a conserved proregion and a variable C-terminal antimicrobial domain. Defensins are the best-characterized AMPs, they have six invariant cysteines, forming three intramolecular cystine-disulfide bonds.
Mode of action
The mode of action of AMPs elucidated to date include inhibition of cell wall formation, formation of pores in the cell membrane resulting in the disruption of membrane potential with eventual lysis of the cell. These peptides also inhibit nuclease activity of both RNase and DNase.
Functions
They have a broad ability to kill microbes. AMPs form an important means of host defense in eukaryotes. Large AMPs (>100 amino acids), are often lytic, nutrient-binding proteins or specifically target microbial macromolecules. Small AMPs act by disrupting the structure of microbial cell membranes. It plays an active role in wound repair and regulation of the adaptive immune system. They have multiple roles as mediators of inflammation with impact on epithelial and inflammatory cells, influencing diverse processes such as cell proliferation, wound healing, cytokine release, chemotaxis and immune induction 3.
References
1. Gottlieb CT, Thomsen LE, Ingmer H, Mygind PH, Kristensen HH, Gram L(2008). Antimicrobial peptides effectively kill a broad spectrum of Listeria monocytogenes and Staphylococcus aureus strains independently of origin, sub-type, or virulence factor expression. BMC Microbiol., 8:205.
2. Yeaman MR and Yount NY (2003). Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmocological Reviews, 55(1).
3. Hanna Galkowska H and Olszewski WL (2003). Antimicrobial peptides – their role in immunity and therapeutic potential. Centr Eur J Immunol., 28 (3):138–141.
抗菌肽介绍二
Ribosomally synthesized antimicrobial peptides (AMPs) constitute a structurally diverse group of molecules found virtually in all organisms. Most antimicrobial peptides contain less than 100 amino acid residues, have a net positive charge, and are membrane active. They are major players in the innate immune defense but can also have roles in processes as chemokine induction, chemotaxis, inflammation, and wound healing. In addition to their antimicrobial effects, many of them show antiviral and antineoplastic activities.
INTRODUCTION
AMPs are a heterogeneous group of relatively small molecules usually containing less than a hundred amino acids. They were first described in the 1960’s by Zeya and Spitznagel in polymorphonuclear leukocyte lysosomes.
To date, more than 2600 AMPs have been identified and registered in databases. They are produced by nearly all groups of organisms, including bacteria, fungi, plants, and animals. Many vertebrate AMPs are secreted by epithelial surfaces such as the tracheal, lingual, or intestinal mucosa of mammals or the skin of amphibia. Some are expressed in neutrophils, monocytes, and macrophages.
AMPs are involved in both animal and plant immune defense systems. Constitutively expressed or induced they play a key role in the first line of defense against microbial intruders.
STRUCTURE/CLASSIFICATION
AMPs can be classified on the basis of their amino acid composition and structure. Two major groups of AMPs can be distinguished. The first group consists of linear molecules which either tend to adopt α-helical structure or are enriched in certain amino acids such as arginine, glycine, histidine, proline, and tryptophan. The second group consists of cysteine-containing peptides which can be divided into single or multiple disulfide structures. In many cases, the presence of disulfide bridges is required for antimicrobial activity.
Most AMPs are cationic peptides, but there are also anionic peptides such as dermcidin, an aspartic acid-rich peptide from human and maximin H5 from amphibian skin. Other non-cationic AMPs include fragments from neuropeptide precursor molecules such as proenkephalin A, aromatic dipeptides primarily isolated from dipteran larvae, or peptides derived from oxygen-binding proteins from arthropod or annelid species.
MODE OF ACTION
Most AMPs act by provoking an increase in plasma membrane permeability. They preferentially target microbial versus mammalian cells. Selectivity is influenced by several factors such as differences in membrane composition: membranes of many bacterial pathogens contain negatively charged lipid moieties such as phosphatidylglycerol (PG), cardiolipin, and phosphatidylserine (PS), whereas mammalian membranes, commonly enriched in phosphatidylethanolamine (PE), phosphatidylcholine (PC) and sphingomyelin, are generally neutral in net charge.
The presence of sterols such as cholesterol and ergesterol within the membrane may be a further means by which AMPs can distinguish between mammalian or fungal cells and prokaryotes. A first step in the mechanism of membrane permeabilization is the electrostatic interaction between the positively charged AMP with the negatively charged membrane surface of the microorganism. Subsequent disruption of the membrane by creation of pores within the microbial membrane ultimately results in cell death of the organism due to leakage of ions, metabolites, cessation of membrane-coupled respiration, and biosynthesis.
Several models for pore formation such as the Barrel-Stave, the Toroidal or Wormhole Model, and the Carpet Model have been proposed (Fig. 1).
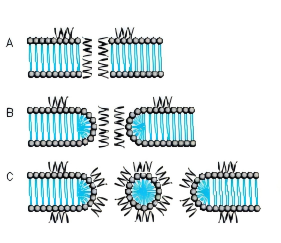
FIG. 1. MODE OF ACTION A BARREL-STAVE MODEL B TOROIDAL PORE OR WORMHOLE MODEL C CARPET MODEL
THE BARREL-STAVE MODEL
The Barrel-Stave model describes a mechanism in which AMPs form a barrellike pore within the bacterial membrane with the individual AMPs or AMP complexes being the staves. Arranged in this manner, the hydrophobic regions of the AMPs point outwards towards the acyl chains of the membrane whereas the hydrophilic areas form the pore.
THE TOROIDAL PORE OR WORMHOLE MODEL
The pores described by this model differ from those of the Barrel-Stave model. Primarily, the outer and inner leaflet of the membrane are not intercalated in the transmembrane channel.
THE CARPET MODEL
A different mechanism is proposed in the Carpet model where AMPs first cover the outer surface of the membrane and then disrupt the membrane like detergents by forming micelle-like units. Certain AMPs penetrate the bacterial membrane without channel formation. They act on intracellular targets by e.g. inhibiting nucleic acid and/or protein synthesis.
RESISTANCE
Resistance to AMPs can either be constitutive or inducible. Inherited resistance mechanisms include altered surface charge, active efflux, production of peptidases or trapping proteins, and modification of host cellular processes. For instance, Staphylococcus aureus manages to reduce the overall cell surface charge by esterification of the cell wall component teichoic acid with D-alanine and thereby increases its resistance against human AMPs. Another example for changing the surface net charge is the production of cationic lysine-substituted phosphatidylglycerol (L-PG) found in certain Staphylococcus aureus strains. In Gram-negative bacteria, addition of 4-aminoarabinose (Ara4N) to the phosphate group of the lipid A backbone or increased acylation of lipopolysaccharides (LPS) are important mechanisms of AMP resistance. Exposure to AMPs may also induce stress responses by which microorganisms try to survive. Inducible regulatory mechanisms have been described in a variety of organisms. For instance, the PhoP/PhoQ regulon in Salmonella has been demonstrated to regulate transcriptional activation of surface and secretory proteins, enzymes that modify lipopolysaccharide, lipid and protein constituents of the outer membrane and proteases that likely degrade certain AMPs.
EXAMPLES OF ANTIMICROBIAL PEPTIDES
| Cationic peptides enriched for specific amino acids |
|
|---|---|
| Glycine-containing peptides | Hymenoptaecin from honeybees |
| Glycine- and proline-containing peptides | Coleoptericin from beetles Holotricin from beetles |
| Histidine-containing peptides | Histatins from humans and some higher primates |
| Proline-containing peptides | Abaecin from honeybees |
| Proline- and arginine-containing peptides | Apidaecins from honeybees Bactenicins from cattle Drosocin from Drosophila PR-39 from pigs |
| Proline- and phenylalanine-containing peptides | Prophenin from pigs |
| Tryptophan-containing peptides | Indolicidin from cattle |
| Linear cationic α-helical peptides | |
|---|---|
| Andropin from insects Bombinin from amphibians Buforin II from amphibians CAP18 from rabbits Cepropins from insects Cecropin P1 from the pig intestinal parasitic nematode, Ascaris suum Ceratotoxin from insects Dermaseptin from amphibians LL-37 from human Magainin from amphibians Melittin from insects Pleurocidin from Pseudopleuronectes americanus |
| Anionic and cationic peptides that contain cysteine and form disulfide bonds |
|
|---|---|
| 1 Disulfide bond | Brevinins |
| 2 Disulfide bonds | Protegrins from pigs |
| 3 Disulfide bonds | α-Defensins from human, rabbits and rats β-Defensins from humans, cattle, mice, rats, pigs, goats and poultry θ-Defensin from the rhesus monkey Insect defensins (Defensin-A from Aedes aegypti) |
| 4 Disulfide bonds | Antifungal defensins from plants Drosomycin from Drosophila |
| Anionic peptides | Dermcidin from human skin Maximin H5 from amphibian skin |
| Anionic and cationic peptide fragments derived from precursor proteins |
Antimicrobial domains from bovine α-lactalbumin, human hemoglobin, lysozyme, and ovalbumin Aromatic dipeptides from dipteran larvae Casocidin I from human casein Enkelytin from proenkaphalin A Lactoferricin from lactoferrin |
ADAPTED FROM K.A. BROGDEN, NAT. REV. MICROBIOL. 3, 238-250 (2005)
IMPORTANT FAMILIES OF AMPS
BOMBININS
Bombinins constitute a family of AMPs produced in fire-bellied toads (Bombina species) active against Gram-negative and Gram-positive bacteria and fungi. Bombinins, bombinin-like peptides (BLPs), and Bombinin H molecules are found in the species Bombina bombina, Bombina variegata, and Bombina orientalis, whereas the homologous maximins and maximin H peptides are derived from the giant fire-bellied toad Bombina maxima. Bombinin H peptides contain either 17 or 20 amino acid residues and are more hydrophobic than bombinins, some of them contain D-alloisoleucine at position 2. They exhibit lower antibacterial activity than bombinins but, in contrast to them, they possess haemolytic activity.
CATHELICIDINS
Members of this family are amphipathic, cationic peptides with a broad-spectrum antimicrobial activity. Cathelicidins typically act by disrupting the integrity of bacterial membranes. They are characterized by an evolutionary conserved N-terminal cathelin- like domain of approximately 99-114 amino acid residues linked to a C-terminal antimicrobial domain of 12-100 residues that can be released upon proteolytic processing. Members of this family include linear peptides amongst them a number of proline-rich AMPs that show different types of proline repeat motifs (Bac5, Bac7, PR-39, prophenins) and the tryptophan-rich indolicidin characterized by three regularly spaced proline residues. The protegrins (PG-1 to PG-5) contain two disulfide bridges and an amidated C-terminus. Cathelicidins have been found in every mammalian species examined. In human, LL-37 (Product 4042456) is the only member of the cathelicidin family. The peptide consists of 37 amino acids and contains two leucine residues at the N-terminus. It is proteolytically cleaved from the 18 kDa precursor protein human cathelicidin antimicrobial protein CAP-18. LL-37 is primarily produced by phagocytic leucocytes and epithelial cells, and is involved in various processes such as direct killing of microorganisms, binding and neutralizing LPS, chemotaxis and chemokine induction, regulation of inflammatory responses, and wound healing. Its production is influenced by several factors such as microbial products, host cytokines, vitamin D3, and availability of oxygen. LL-37 orthologues in mouse and rat are CRAMP (mouse) (Product 4056438) and CRAMP (rat), respectively.
CECROPINS
Cecropins were first isolated from the giant silk moth Hyalophora cecropia. They can form amphipathic, α-helical structures and are structurally related to other cecropins as bactericidin, lepidopteran, and sarcotoxin. Cecropin P1 (Product 4039862), found in pig intestine, also belongs to this family. Most cecropins show broad-spectrum antibacterial activity. Cecropin A (Product 4030488) and B (Product 4030477) have also been demonstrated to possess tumoricidal activity against mammalian leukemia, lymphoma, and carcinoma cell lines.
CERATOTOXINS
This family consists of cationic α-helical amphipathic peptides expressed in the female reproductive accessory glands of the Mediterranean fruit fly Ceratitis capitata. The production of the peptides is enhanced by mating. Ceratotoxin A and ceratotoxin B are 29 amino acid peptides differing in two amino acids. Ceratotoxin C and D consist of 32 and 36 amino acids, respectively. The peptides of this family are active against Gram-negative as well as Grampositive bacteria and are supposed to act via the Barrel-Stave model. Ceratotoxin A has been shown to be mainly antibacterial for Gram-negative organisms.
DEFENSINS
Defensins are small cysteine-rich cationic peptides containing three or four disulfide bridges. They have been isolated from molluscs, acari, arachnids, insects, mammals, and plants. They are further divided into families on the basis of the spatial distribution of their cysteine residues. Three families, the α-, β- and θ-defensins, can be distinguished in mammals. α- and β-defensins are characterized by antiparallel β-sheet structures stabilized by three disulfide bonds. The θ-defensins are found in rhesus monkey and some other non-human primates but not in human, chimpanzee and gorilla. They consist of two nine amino acid peptides derived from different precursor proteins joined by head-to-tail cyclization. Invertebrate and plant defensins contain three or four disulfide bridges, respectively. Insect and mammalian defensins are mainly active against bacteria while most plant defensins possess antifungal activity.
DERMASEPTINS
The peptides of the dermaseptin family are closely related and consist of 28-34 amino acids. They were originally isolated from skin extracts of the South American arboreal frog Phyllomedusa sauvagei and contain a conserved tryptophan residue at position 3. Dermaseptins exhibit broad-spectrum antimicrobial activity against Gram-positive and Gram-negative bacteria.
HISTATINS
Histatins are histidine-rich and mostly cationic peptides found in the saliva of humans and some higher primates. They are active against a broad-spectrum of bacteria and fungi. The antifungal activity of the human salivary peptide histatin-5 has been extensively studied and is supposed to be due to inhibition of mitochondrial respiration and the formation of reactive oxygen species. Histatin-5 has also been shown to inhibit both host-derived and bacterial proteolytc enzymes involved in peridontal diseases. Histatin-8, a peptide from human parotid secretion, has been shown to inhibit hemagglutination activity of Porphyromonas gingivalis 381, a Gram-negative bacterium involved in certain forms of periodontal disease. The peptide may function as a binding domain for the hemagglutinins of Porphyromonas gingivalis during agglutination.
MAGAININS
Magainins constitute a family of linear amphipathic cationic AMPs discovered in the skin of Xenopus laevis. The two closely related members of this family, magainin I (Product 4012844) and magainin II (Product 4013706) differ merely in two positions and are 23 amino acids in length. Magainins exhibit broad-spectrum antimicrobial activity against Gram-negative and Gram-positive bacteria, fungi and protozoa and are also cytotoxic for many murine and human cancer cell lines.
CONCLUSIONS
The structures of AMPs represent a unique source for the targeted exploration of new applications in the therapy of microbial and viral infection, cancer, and sepsis. Modern synthetic methods will allow the relatively cheap and accurate production of lead compounds and peptide candidates. The achievements in peptide library generation, analytical methods as mass spectrometry, and screening and formulation technologies may contribute to solve intrinsic problems associated with the use of AMPs as therapeutic agents such as susceptibility to proteases and host toxicity. Bachem has considerable expertise and long-standing experience in peptide synthesis. With our capacity to upscale the production of simple and modified peptides, we are the partner of choice for the pharmaceutical industries.
| DOI | 名称 | |
|---|---|---|
| 10.1099/0022-1317-83-5-1173 | Mutational analysis of a human immunodeficiency virus type 1 Tat protein transduction domain which is required for delivery of an exogenous protein into mammalian cells | 下载 |
| 10.1016/j.ab.2010.09.020 | A modified fluorescent intercalator displacement assay for RNA ligand discovery | 下载 |
| 10.1155/2019/6315954 | Synthesis and Preclinical Evaluation of the Fibrin-Binding Cyclic Peptide 18F-iCREKA: Comparison with Its Contrasted Linear Peptide | 下载 |
| 10.1016/j.addr.2016.08.004 | Functional peptides for siRNA delivery | 下载 |
多肽H2N-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg-COOH的合成步骤:
1、合成CTC树脂:称取2.45g CTC Resin(如初始取代度约为0.48mmol/g)和1.41mmol Fmoc-Arg(Pbf)-OH于反应器中,加入适量DCM溶解氨基酸(需要注意,此时CTC树脂体积会增大好几倍,避免DCM溶液过少),再加入3.53mmol DIPEA(Mw:129.1,d:0.740g/ml),反应2-3小时后,可不抽滤溶液,直接加入1ml的HPLC级甲醇,封端半小时。依次用DMF洗涤2次,甲醇洗涤1次,DCM洗涤一次,甲醇洗涤一次,DCM洗涤一次,DMF洗涤2次(这里使用甲醇和DCM交替洗涤,是为了更好地去除其他溶质,有利于后续反应)。得到 Fmoc-Arg(Pbf)-CTC Resin。结构图如下:
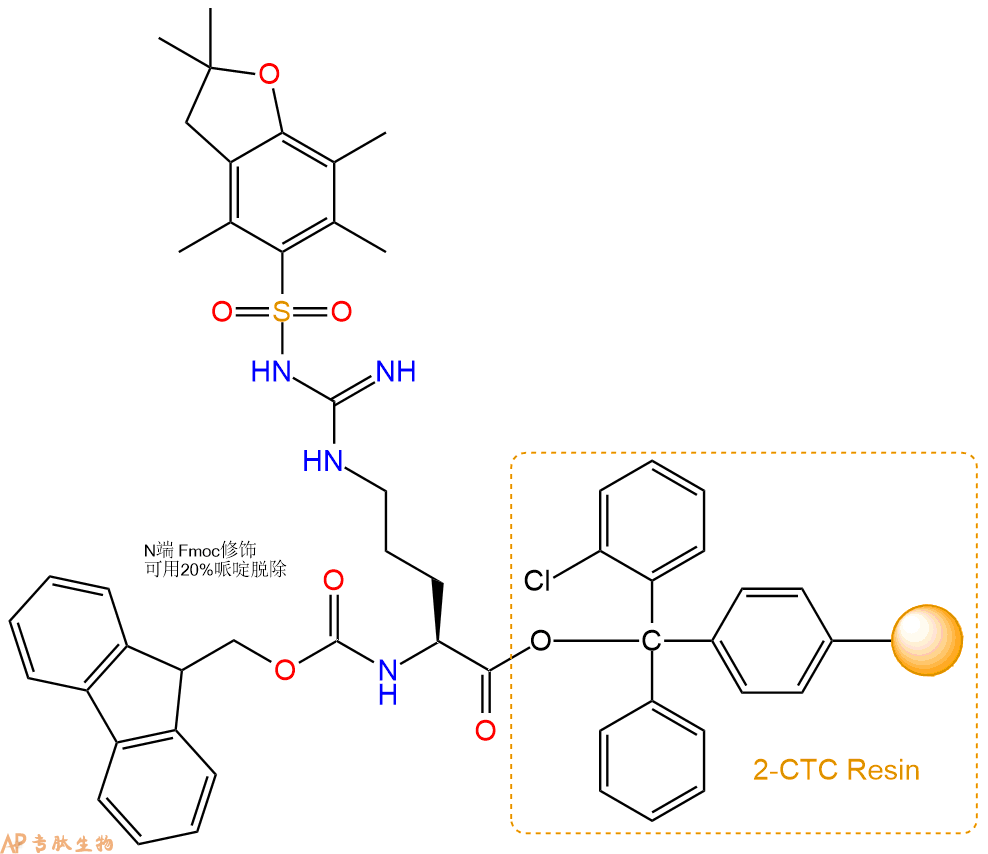
2、脱Fmoc:加3倍树脂体积的20%Pip/DMF溶液,鼓氮气30分钟,然后2倍树脂体积的DMF 洗涤5次。得到 H2N-Arg(Pbf)-CTC Resin 。(此步骤脱除Fmoc基团,茚三酮检测为蓝色,Pip为哌啶)。结构图如下:
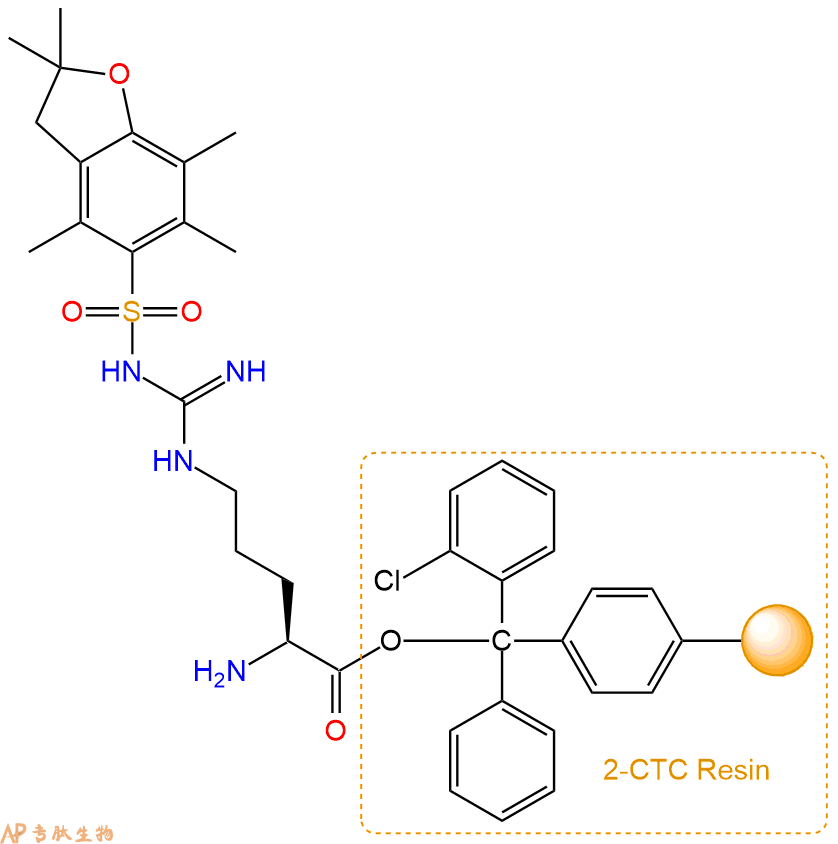
3、缩合:取3.53mmol Fmoc-Arg(Pbf)-OH 氨基酸,加入到上述树脂里,加适当DMF溶解氨基酸,再依次加入7.06mmol DIPEA,3.35mmol HBTU。反应30分钟后,取小样洗涤,茚三酮检测为无色。用2倍树脂体积的DMF 洗涤3次树脂。(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。得到Fmoc-Arg(Pbf)-Arg(Pbf)-CTC Resin。氨基酸:DIPEA:HBTU:树脂=3:6:2.85:1(摩尔比)。结构图如下:
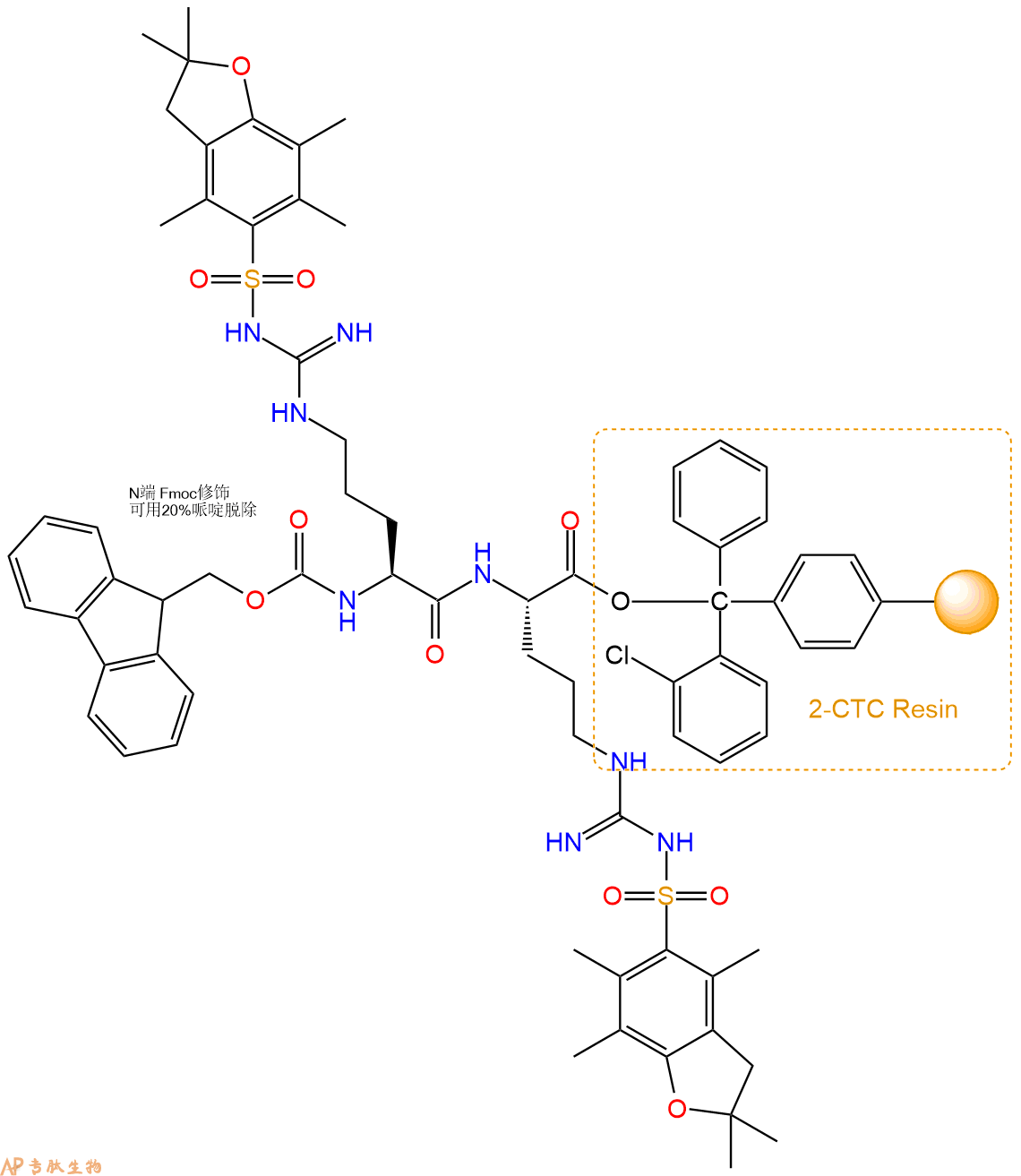
4、依次循环步骤二、步骤三,依次得到
H2N-Arg(Pbf)-Arg(Pbf)-CTC Resin
Fmoc-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-CTC Resin
H2N-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-CTC Resin
Fmoc-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-CTC Resin
H2N-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-CTC Resin
Fmoc-Arg(Pbf)-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-CTC Resin
H2N-Arg(Pbf)-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-CTC Resin
Fmoc-Arg(Pbf)-Arg(Pbf)-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-CTC Resin
H2N-Arg(Pbf)-Arg(Pbf)-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-CTC Resin
Fmoc-Lys(Boc)-Arg(Pbf)-Arg(Pbf)-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-CTC Resin
H2N-Lys(Boc)-Arg(Pbf)-Arg(Pbf)-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-CTC Resin
Fmoc-Lys(Boc)-Lys(Boc)-Arg(Pbf)-Arg(Pbf)-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-CTC Resin
H2N-Lys(Boc)-Lys(Boc)-Arg(Pbf)-Arg(Pbf)-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-CTC Resin
Fmoc-Arg(Pbf)-Lys(Boc)-Lys(Boc)-Arg(Pbf)-Arg(Pbf)-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-CTC Resin
以上中间结构,均可在专肽生物多肽计算器-多肽结构计算器中,一键画出。
最后再经过步骤二得到 H2N-Arg(Pbf)-Lys(Boc)-Lys(Boc)-Arg(Pbf)-Arg(Pbf)-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-CTC Resin,结构如下:
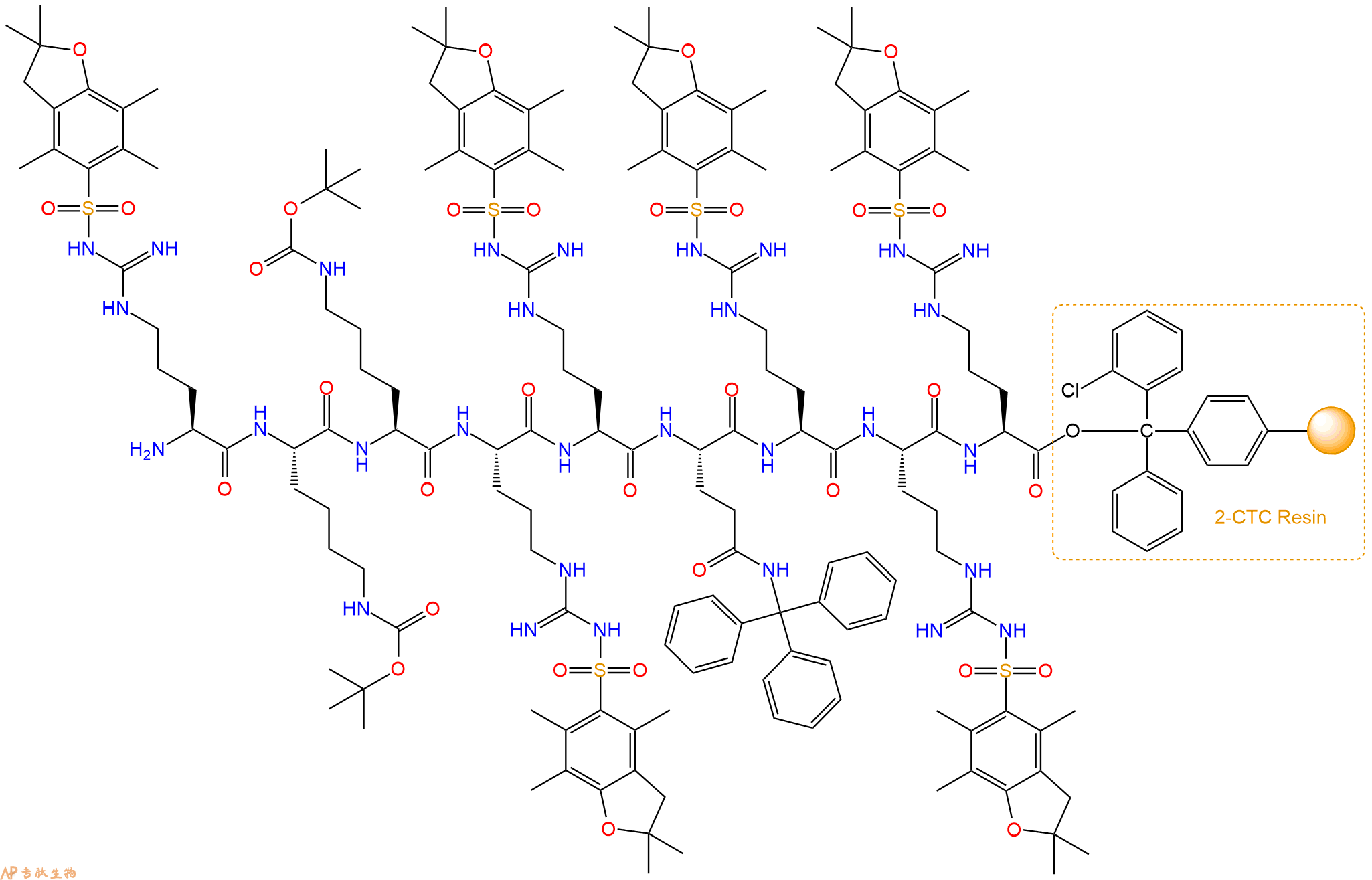
5、切割:6倍树脂体积的切割液(或每1g树脂加8ml左右的切割液),摇床摇晃 2小时,过滤掉树脂,用冰无水乙醚沉淀滤液,并用冰无水乙醚洗涤沉淀物3次,最后将沉淀物放真空干燥釜中,常温干燥24小试,得到粗品H2N-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg-COOH。结构图见产品结构图。
切割液选择:1)TFA:H2O=95%:5%、TFA:H2O=97.5%:2.5%
2)TFA:H2O:TIS=95%:2.5%:2.5%
3)三氟乙酸:茴香硫醚:1,2-乙二硫醇:苯酚:水=87.5%:5%:2.5%:2.5%:2.5%
(前两种适合没有容易氧化的氨基酸,例如Trp、Cys、Met。第三种适合几乎所有的序列。)
6、纯化冻干:使用液相色谱纯化,收集目标峰液体,进行冻干,获得蓬松的粉末状固体多肽。不过这时要取小样复测下纯度 是否目标纯度。
7、最后总结:
杭州专肽生物技术有限公司(ALLPEPTIDE https://www.allpeptide.com)主营定制多肽合成业务,提供各类长肽,短肽,环肽,提供各类修饰肽,如:荧光标记修饰(CY3、CY5、CY5.5、CY7、FAM、FITC、Rhodamine B、TAMRA等),功能基团修饰肽(叠氮、炔基、DBCO、DOTA、NOTA等),同位素标记肽(N15、C13),订书肽(Stapled Peptide),脂肪酸修饰肽(Pal、Myr、Ste),磷酸化修饰肽(P-Ser、P-Thr、P-Tyr),环肽(酰胺键环肽、一对或者多对二硫键环),生物素标记肽,PEG修饰肽,甲基化修饰肽
以上所有内容,为专肽生物原创内容,请勿发布到其他网站上。





