400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研
Prion Protein 106-126 (human) 是 Prion 的多肽片段,它可以诱导神经元凋亡,抗蛋白酶 K 消化,纤维形成,并介导正常细胞病毒蛋白 (PrPc) 转化为致病同工型 (PrPSc)
编号:200308
CAS号:148439-49-0
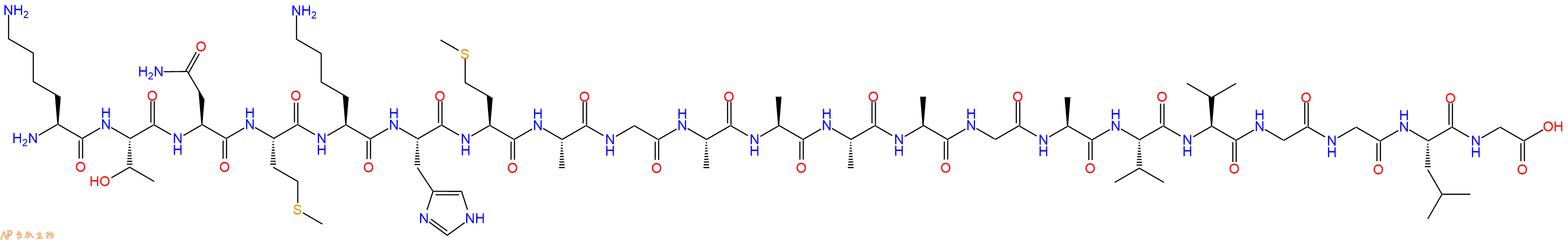
单字母:H2N-KTNMKHMAGAAAAGAVVGGLG-OH
Prion Protein 106-126 (human) 是 Prion 的多肽片段,它可以诱导神经元凋亡,抗蛋白酶 K 消化,纤维形成,并介导正常细胞病毒蛋白 (PrPc) 转化为致病同工型 (PrPSc)。Prion Protein 106-126 (human) 通常用作研究朊病毒蛋白疾病中神经变性的模型。
Prion Protein 106-126 (human), a peptide fragment of prion, and can induct neuronal apoptosis, antiproteinase K digestion, fiber formation, and mediate the conversion of normal cellular prion protein (PrPc) into pathogenic isoform (PrPSc). Prion Protein 106-126 (human) is generally used as the model to investigate neural degeneration of prion disease.
朊病毒蛋白(PrP),也称为CD230,主要在神经系统中产生。PrP以不同的亚型存在,其中包括正常PrPc和“羊瘙痒病”亚型PrPSc。PrPSc折叠错误,与多种疾病有关,包括牛海绵状脑病、Gerstmann-Straüssler-Scheindker病(GSS)和克雅氏病。它含有比PrPc高得多的β片含量,并倾向于形成抗蛋白酶的聚集体。支持这些聚集体的促凝作用及其在神经退行性疾病中作用的一个假设是,由其与PrPSc的相互作用引发的PrPc从正常变为PrPSc(构象转换假说)。
Prion protein (PrP), also known as CD230, is mainly produced in the nervous sytem. PrP exists as different isoforms, among which the normal PrPc, and the 'scrapie' isoform PrPSc. PrPSc is misfolded and associated to multiple disorders including bovine spongiform encephalopathy, Gerstmann-Straüssler-Scheindker disease (GSS) and the Creutzfeldt-Jakob disease. It contains a much higher beta-sheet content than PrPc, and tends to form protease-resistant aggregates. A hypothesis to support the propoagation of these aggregates and their role in neurodegeneration is the change from normal PrPc to PrPSc triggered by its interaction with PrPSc (conformation conversion hypothesis).
PrP 127-147形成丝状结构,压迫羊瘙痒病相关纤维,但其淀粉样蛋白生成潜力低于PrP 106-126。
PrP 127-147 forms filamentous structures ressembling scrapie-associated fibrils, but possesses a lower amyloidogenic potential than PrP 106-126.
这种肽增加了循环白细胞的膜微粘度、细胞内Ca2+浓度和细胞迁移,以及单核细胞和中性粒细胞的氧气产生。它还诱导GH3细胞系中的凋亡细胞死亡和L型电压敏感钙通道活性的损伤。PrP(106-126)以剂量依赖的方式刺激白细胞迁移。
This peptide increases membrane microviscosity, intracellular Ca2+ concentration and cell migration in circulating leucocytes, and oxygen production in monocytes and neutrophils. It also induces apoptotic cell death and impairment of L-type voltage-sensitive calcium channel activity in the GH3 cell lines. PrP (106–126) stimulates leucocyte migration in a dose-dependent manner.
Forloni G, et, al. Neurotoxicity of a prion protein fragment. Nature. 1993 Apr 8; 362(6420): 543-6. : https://pubmed.ncbi.nlm.nih.gov/8464494/
Hong JM, et, al. Human prion protein-mediated calcineurin activation induces neuron cell death via AMPK and autophagy pathway. Int J Biochem Cell Biol. 2020 Feb; 119: 105680. : https://pubmed.ncbi.nlm.nih.gov/31866508/





