| 编号: | 194123 |
| 中文名称: | 血管生成素Angiogenin Fragment (108-122) |
| 英文名: | Angiogenin Fragment (108-122) |
| CAS号: | 112173-49-6 |
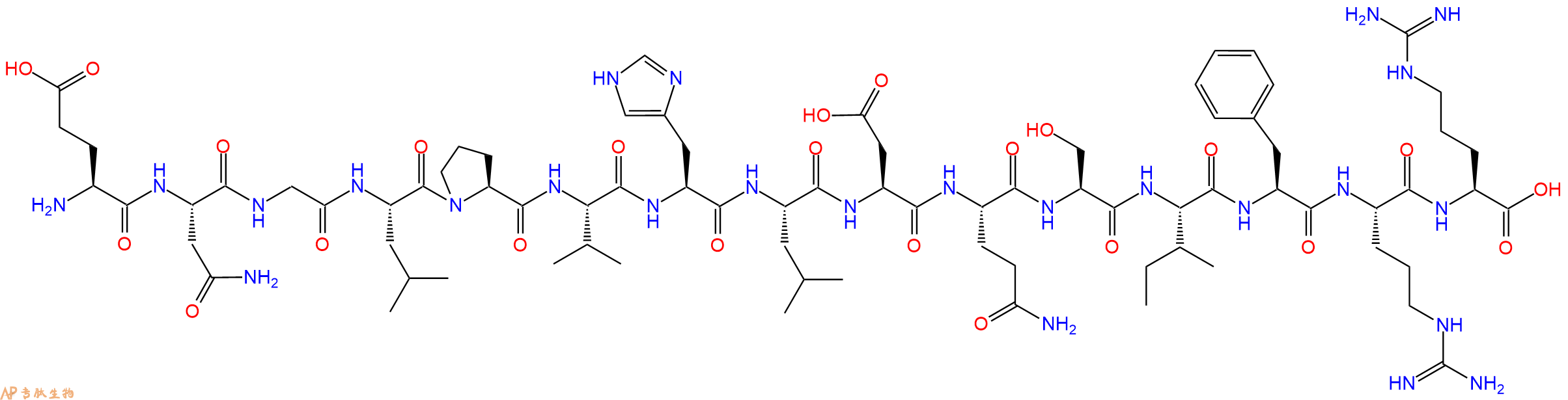
| 单字母: | H2N-ENGLPVHLDQSIFRR-OH |
| 三字母: | H2N N端氨基 -Glu谷氨酸 -Asn天冬酰胺 -Gly甘氨酸 -Leu亮氨酸 -Pro脯氨酸 -Val缬氨酸 -His组氨酸 -Leu亮氨酸 -Asp天冬氨酸 -Gln谷氨酰胺 -Ser丝氨酸 -Ile异亮氨酸 -Phe苯丙氨酸 -Arg精氨酸 -Arg精氨酸 -OHC端羧基 |
| 氨基酸个数: | 15 |
| 分子式: | C78H125N25O23 |
| 平均分子量: | 1780.98 |
| 精确分子量: | 1779.94 |
| 等电点(PI): | 10.4 |
| pH=7.0时的净电荷数: | 1.21 |
| 平均亲水性: | 0.21538461538462 |
| 疏水性值: | -0.66 |
| 外观与性状: | 白色粉末状固体 |
| 消光系数: | - |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 纯度: | 95%、98% |
| 盐体系: | 可选TFA、HAc、HCl或其它 |
| 生成周期: | 2-3周 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 血管紧张素(Angiotensin) 血管生成素(Angiogenin、ANG) |
Angiogenin (108-122) 是一种血管生成素肽。
Angiogenin (108-122) is an angiogenin peptide.
定义
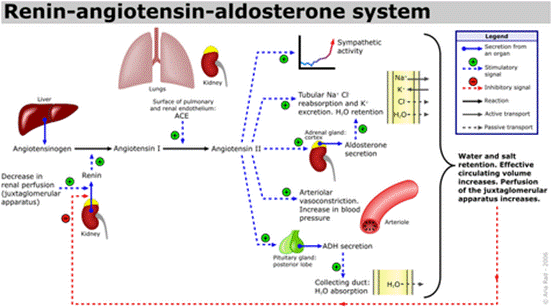
血管生成素(ANG),一种14,124 Da的蛋白质,与肿瘤进展中的血管生成有关。它由肿瘤细胞分泌,肿瘤细胞是新生血管形成的有效诱导剂1。同时血管生成素可引起血管收缩并促使血压升高。它是肾素-血管紧张素系统的一部分,这是降低血压的药物的主要靶标。血管紧张素(ANG)是血管紧张素原(AGT)在肾素和一系列酶的作用下形成的一组寡肽激素。
血管紧张素是一种肽类激素,引起血管收缩及随后血压升高。血管紧张素是一种寡肽类激素,具有强大的致渴作用。它是由前体分子血管紧张素原生成的,血管紧张素原是肝脏中产生的血清球蛋白。血管紧张素在肾素-血管紧张素系统中起重要作用(1)。肾素作用于血管紧张素原,产生血管紧张素I。在肾小球旁细胞中,当肾交感神经兴奋、肾内血压降低或运送到致密斑的Na+和Cl-离子降低,致密斑感受到较少的Na+时,肾小球旁细胞释放的肾素增加。肾素裂解血管紧张素原中亮氨酸(Leu)和缬氨酸(Val)之间的肽键,生成含有十个氨基酸的多肽(DES-ASP),即血管紧张素I,血管紧张素I似乎没有任何的生物活性,仅仅是作为血管紧张素II的前体。
发现
1885年,Fett等人首先从由HT-29人结肠腺癌细胞1调节的培养基中分离血管生成素,然后从正常哺乳动物的血浆2和牛奶中分离出血管生成素。随后已从人,牛,兔,猪和小鼠的血清和牛乳中分离出它。血管生成素具有核糖核酸分解活性,与胰腺RNAse A 具有33%的序列同源性。合成肽'H-Glu-Asn-Gly-Leu-Pro-Val-His-Leu-Asp-Gln-Ser-Ile-Phe-Arg-Arg-OH(108-122)对应于ANG抑制血管生成素的酶和生物活性。几种C末端合成肽,包括(Ang 108-123),可显着降低血管生成素诱导的新血管形成。
结构特点
Acharya等人在2.4 0 A时确定了人类抗原的晶体结构。总体结构具有肾形三级褶皱,让人联想到RNaseA。核糖核酸裂解活性中心(His-13,His-114和Lys-40)和假定的受体结合位点,这两个关键参与生物学功能是不同于Rnase A 。 分子的中心核心由带有一对反平行扭曲的P结构组成,形成了主拓扑,顶点处有残基Ser-72和Gly-99。在这些中央链的任一侧的两个附加链(残基41-47; 111-116)完成了主要的片状结构。结构中存在3个螺旋H1,残基3-14,H2,残基22-33和H3,残基49-58。
作用机理
ANG四个方面已经发现,是必要的ANG诱导的血管生成的过程中,ANG发挥其核糖核酸活动- ANG有一个非常弱的10 5 - 10 6比核糖核酸酶答:这更低的核糖核酸活动,因为嘧啶结合谷氨酰胺(Gln)117残基“阻碍”了ANG的位点。然而,ANG的核糖核酸分解活性对于血管生成至关重要。ANG提神基底膜降解- ANG结合到一个上肌动蛋白内皮细胞表面 ANG-肌动蛋白复合物从细胞表面解离并加速组织型纤溶酶原激活物(tPA)催化的纤溶酶从纤溶酶原的生成。ANG-肌动蛋白复合物促进基底膜和细胞外基质的降解。该复合物允许内皮细胞渗透并迁移到血管周组织中。基底膜降解是血管生成的基本特征。ANG激活信号转导- ANG结合至170-kDa的 位于内皮细胞表面上的受体和引发第二信使系统。ANG与细胞表面肌动蛋白的结合导致细胞相关蛋白酶系统的活化,从而促进细胞侵袭。ERK1 / 2,蛋白激酶B / Akt1 已经提出通过ANG刺激来激活这些途径。ANG核易位-血管生成素通过受体介导的内吞作用和核定位序列辅助的核输入在内皮细胞中进行核易位。核定位信号(NLS)位于蛋白质的31-RRRGL-35中。核易位后,它增强rRNA转录。
功能
血管紧张素受体存在于许多组织类型中,包括肾上腺皮质,肾小球,心脏,下丘脑,肝脏,胰腺,垂体,血小板,肾小管,子宫和血管平滑肌。通过放射性配体与拮抗剂的结合已鉴定出两种高亲和力受体亚型:氯沙坦(DuP 753 / MK954)鉴定AT1受体;PD123177和CGP42112A是AT2受体的标记。血管紧张素II可能在体液系统外的组织中局部产生。例如,它存在于大脑,肾脏和心脏。在大脑中,七肽血管紧张素(1-7)模仿血管紧张素II的某些作用,但可能直接由血管紧张素I形成。心脏中有非ACE介导的血管紧张素II产生的证据。血管内血管紧张素II受体与血管加压素和其他垂体后叶激素的中枢释放,交感神经外流增加,口渴反应以及可能的认知功能有关。血管紧张素II对心脏以及对生长/肥大的正性和变时性作用; 控制醛固酮的释放以及皮质醇和醛固酮分泌之间的平衡;并调节钠,氯和碳酸氢盐在肾脏内的运输。
ANG在血管生成中的功能-作为关键的血管生成因子,ANG与内皮细胞和平滑肌细胞相互作用,诱导多种细胞应答,包括细胞迁移,侵袭,增殖和肾小管结构形成。还已经报道ANG可直接诱导癌细胞的增殖。最近,ANG 基因被确定为潜在的肌萎缩性侧索硬化症(ALS)相关基因6。ANG可诱导多种人类癌症中的肿瘤生长,包括乳腺癌,宫颈癌,结肠癌,结肠直肠癌,子宫内膜癌,胃癌,肝癌,肾癌,卵巢癌,胰腺癌,前列腺癌和尿路上皮癌,以及星形细胞瘤,白血病,淋巴瘤,黑素瘤,骨肉瘤,和肾母细胞“肿瘤 6。ANG可能与肌萎缩性侧索硬化症有关-肌萎缩性侧索硬化症(ALS)是一种进展性迟发性神经退行性疾病,会影响上下运动神经元(MNs)。血管内皮生长因子是第一个被证明与ALS 7发病有关的血管生成因子。
Definition
Angiogenin (ANG), a 14,124 Da protein that has been implicated in angiogenesis in tumor progression. It is secreted by tumor cells, a potent inducer of neovascularization1.
Discovery
In 1885, Fett et al., first isolated and characterized angiogenin from medium conditioned by HT-29 human colon adenocarcinoma cells1 and later from normal mammalian plasma2 and milk. It has been subsequently isolated from human, bovine, rabbit, pig & mouse sera and bovine milk. Angiogenin has ribonucleolytic activity with 33% sequence homology to pancreatic RNAse A3. A synthetic peptide ’H-Glu-Asn-Gly-Leu-Pro-Val-His-Leu-Asp-Gln-Ser-Ile-Phe-Arg-Arg-OH (108-122) corresponding to the C-terminal region of ANG inhibits the enzymatic and biological activities of angiogenin4. Several C-terminal synthetic peptides, including (Ang 108-123), significantly decreases angiogenin-induced neovascularization.
Structural characteristics
Acharya et al., determined the crystal structure of human antigenic at 2.4 0A. Overall Structure features a kidney shaped tertiary fold reminiscent of RNase A. The ribonucleolytic active center (His-13, His-114, and Lys-40) and the putative receptor binding site, both of which are critically involved in biological functions are distinct from Rnase A5. The central core of the molecule consists of P structure with a pair of antiparallel twisted forming the main topology with residues Ser-72 and Gly-99 at the apices. Two additional strands on either side of these central strands (residues 41-47; 111-116) complete the major sheet structure. There are 3 helices H1, residues 3-14, H2, residues 22-33 and H3, residues 49-58 present in the structure.
Mechanism of action
Four aspects of ANG have been discovered that is necessary for the process of ANG-induced angiogenesis,ANG exerts its ribonucleolytic activity-ANG has a very weak 105-106 lower ribonucleolytic activity than that of RNase A. This is because the pyrimidine binding site of ANG is “obstructed” by the glutamine (Gln)117 residue. However, ribonucleolytic activity of ANG is crucial for angiogenesis.ANG stimulates basement membrane degradation-ANG binds to a-actin on endothelial cell surface; ANG-actin complexes dissociate from the cell surface and accelerate tissue type plasminogen activator (tPA)-catalyzed generation of plasmin from plasminogen. ANG-actin complexes promote the degradation of basement membrane and extracellular matrix. This complex allows endothelial cells to penetrate and migrate into the perivascular tissue. Basement membrane degradation is an essential feature of angiogenesis. ANG activates signaling transduction- ANG binds to a 170-kDa receptor located on the endothelial cell surface and elicits second messenger systems. Binding of ANG to cell surface actin results in activation of a cell-associated protease system that promotes cell invasion. ERK1/2, protein kinase B/Akt1 pathways have been proposed to be activated by ANG stimulation. ANG nuclear translocation-Angiogenin undergoes nuclear translocation in endothelial cells via receptor-mediated endocytosis and nuclear localization sequence-assisted nuclear import. A nuclear localization signal (NLS), lies in 31-RRRGL-35 of the protein. Upon nuclear translocation it enhances rRNA transcription.
Functions
Functions of ANG in Angiogenesis-As a key angiogenic factor, ANG interacts with endothelial and smooth muscle cells to induce a wide range of cellular responses including cell migration, invasion, proliferation, and formation of tubular structures. ANG has also been reported to induce the proliferation of cancer cells directly. Recently, ANG gene was identified to be a potential amyotrophic lateral sclerosis (ALS) related gene6. ANG induces tumor growth in various types of human cancers, including breast, cervical, colon, colorectal, endometrial, gastric, liver, kidney, ovarian, pancreatic, prostate, and urothelial cancers, as well as astrocytoma, leukemia, lymphoma, melanoma, osteosarcoma, and Wilms’ tumor 6. ANG may be related with amyotrophic lateral sclerosis- Amyotrophic lateral sclerosis (ALS) is a progressive late onset neurodegenerative disorder affecting upper and lower motoneurons (MNs). Vascular endothelial growth factor was the first angiogenic factor shown to contribute to the pathogenesis of ALS 7.
References
1. Fett JW, Strydom DJ, Lobb RR, Alderman EM, Bethune JL, Riordan JF, Vallee BL (1985). Isolation and characterization of angiogenin, an antigenic protein from human carcinoma cells. Biochemistry, 24: 5480-5486.
2. Shapiro R, Strydom DJ, Olson KA, Vallee BL (1987). Isolation of angiogenin from normal human plasma. Biochemistry, 26: 5141-5146.
3. Strydom DJ, Fett JW, Lobb RR, Alderman EM, Bethune JL, Riordan JF, Vallee BL (1985). Amino acid sequence of human tumor derived angiogenin. Biochemistry, 24(20): 5486–5494.
4. Rybak SM, Auld DS, St Clair DK, Yao QZ, Fett JW (1989). C-terminal angiogenin peptides inhibit the biological and enzymatic activities of angiogenin. Biochem. Biophys. Res. Commun, 162: 535–543.
5. Acharya KR, Shapiro R, Allen SC, Riordan JF, Vallee BL (1994). Crystal structure of human angiogenin reveals the structural basis for its functional divergence from ribonuclease. Proc Natl Acad Sci USA, 91: 2915-2919.
6. Yoshioka N, Wang L, Kishimoto K, Tsuji T, Hu GF (2006). A therapeutic target for prostate cancer based on angiogenin-stimulated angiogenesis and cancer cell proliferation. Proc Natl Acad Sci USA, 103: 14519-14524.
7. Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van Dorpe J, Hellings P, Gorselink M, Heymans S, Theilmeier G, Dewerchin M, Laudenbach V, Vermylen P, Raat H, Acker T, Vleminckx V, Van Den Bosch L, Cashman N, Fujisawa H, Drost MR, Sciot R, Bruyninckx F, Hicklin DJ, Ince C, Gressens P, Lupu F, Plate KH, Robberecht W, Herbert JM, Collen D, Carmeliet P(2001). Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Gene, 28:131-138.
| DOI | 名称 | |
|---|---|---|
| 10.1016/0006-291x(89)92030-5 | C-terminal angiogenin peptides inhibit the biological and enzymatic activities of angiogenin | 下载 |





