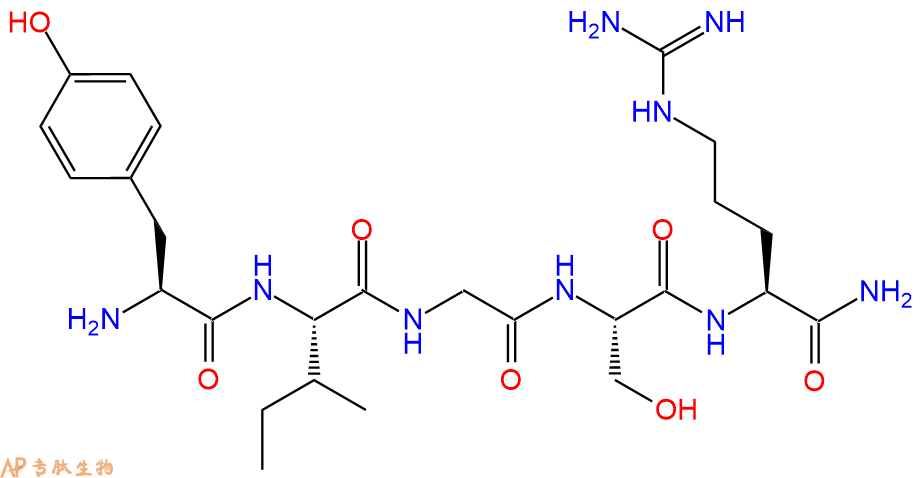
YIGSR-酰胺与层粘连蛋白受体结合并抑制实验性转移形成。与 YIGSR 相比,C 端酰胺化显着增加了肽的活性。
编号:161787
CAS号:110590-65-3
单字母:H2N-YIGSR-NH2
| 编号: | 161787 |
| 中文名称: | Laminin Penta Peptide , amide |
| 英文名: | Laminin Penta Peptide , amide |
| CAS号: | 110590-65-3 |
| 单字母: | H2N-YIGSR-NH2 |
| 三字母: | H2N N端氨基 -Tyr酪氨酸 -Ile异亮氨酸 -Gly甘氨酸 -Ser丝氨酸 -Arg精氨酸 -NH2C端酰胺化 |
| 氨基酸个数: | 5 |
| 分子式: | C26H43N9O7 |
| 平均分子量: | 593.68 |
| 精确分子量: | 593.33 |
| 等电点(PI): | - |
| pH=7.0时的净电荷数: | 2.97 |
| 平均亲水性: | -0.475 |
| 疏水性值: | -0.42 |
| 外观与性状: | 白色粉末状固体 |
| 消光系数: | 1490 |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 纯度: | 95%、98% |
| 盐体系: | 可选TFA、HAc、HCl或其它 |
| 生成周期: | 2-3周 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 层粘连蛋白(Laminins) 整合素家族(Integrins) |
YIGSR-amide binds to the laminin receptor and inhibits experimental metastasis formation. In comparison to YIGSR, C-terminal amidation increases the activity of the peptide significantly.
Laminins are the protein network that is the foundation for most cells and organs. They are large trimeric proteins, also in the family of glycoproteins that contain an alpha chain, a beta chain and a gamma chain which are found in five, three and three genetic variants. As a family of glycoproteins, laminins are an integral part of the structural scaffolding in almost every tissue of an organism. This makes the laminin a vital part of the maintenance and survival of the tissues. Consequently, defective laminins cause muscular dystrophy, junctional epidermolysis bullosa and defects of the kidney a defect.
Recently, publications have shown that laminins can be used to culture cells that are difficult to culture on other substrates. These trimeric glycoproteins form a similar structure to a cross, giving it a structure that has the ability to bind to other cell membrane and extracellular molecules. The three shorter arms of the laminin are particularly efficient at binding to other laminin molecules, which allows them to form larger sheets. The long arm is capable of binding to cells, which then helps anchor organized tissue cells to the membrane. These laminins are an important and biologically active part of the basal lamina, influenincing cell differentiation, migration and adhesion.
Definition
The integrins are a superfamily of cell adhesion receptors that bind to extracellular matrix EMC ligands, cell-surface ligands, and soluble ligands.
Discovery
The discovery of integrins was driven in large part by a series of early observation suggesting that adhesion to ECM is mediated at the cell surface receptors. By 1980s, it became clear that fibronectin was one of the groups of ECM proteins present in the serum that could promote the adhesion of cells to the tissue culture flask.
Structure of integrins
The integrins are a family of alpha, beta heterodimeric receptors. Integrins are expressed by all multicellular animals, but their diversity varies widely among species; for example, in mammals, 19 alpha and 8 beta subunit genes encode polypeptides that combine to form 25 different receptors, whereas the Drosophila and Caenorhabditis genomes encode only five and two integrin alpha subunits respectively.
The N-terminal portion of integrin a subunits comprises seven homologous, tandemly repeated domains of about 50 amino acids. Repeats 4-7 (or in some integrins 5-7) contain cation-binding sequences. Seven integrin a subunits (a1, a2, aE, aL, aM, aX, and aD) contain a domain of ~200 amino acids inserted between the second and third N-terminal repeats. This domain is homologous in sequence to the ‘A’ domains of von Willebrand factor, and has been shown to contain a single cation binding site1
The b ?subunit contains a region of ~240 amino acids near its N terminus that is highly conserved between different b subunits. This region may also have an A-domain-like structure with a cation binding site. The C-terminal portion of the b ?subunit contains a number of cysteine-rich repeats2.
Mechanism of action
Cell-cell and cell-substratum adhesion is mediated by the binding of integrin extracellular domains to diverse protein ligands; however, cellular control of these adhesive interactions and their translation into dynamic cellular responses, such as cell spreading or migration, requires the integrin cytoplasmic tails. These short tails bind to intracellular ligands that connect the receptors to signalling pathways and cytoskeletal networks. Hence, by binding both extracellular and intracellular ligands, integrins provide a transmembrane link for the bidirectional transmission of mechanical force and biochemical signals across the plasma membrane. One important mechanism by which cells regulate integrin function is through tight spatial and temporal control of integrin affinity for extracellular ligands. This is achieved by rapid, reversible changes in the conformation of the extracellular domains of the integrin heterodimer, so-called integrin activation3.
Functions
Proliferation: The mechanism by which integrins control proliferation involves both a direct crosstalk between integrins and growth factor receptors GFRs, and GF- independent signalling from integrins themselves. In some cells, for example, ERK signalling is induced directly by integrin adhesion, whereas the Akt pathway (which also promotes proliferation) can be activated downstream of integrins through mechanisms separate to those of GFRs4.
Apoptosis: Integrins are essential determinants of cell survival and, in many cases, prevention or alteration of integrin adhesion triggers a form of apoptosis that is known as anoikis. Anoikis is particularly relevant when cells become located in ECM environments in which they are not developmentally programmed to reside. For example, mammary epithelial cells are normally situated on a laminin-rich basement membrane but, if they are displaced to a stromal ECM of collagen I, they undergo anoikis5.
Differentiation: For some cell types, the involvement of integrins during their developmental programming to become fully mature, differentiated cells has been extensively characterized. Oligodendrocyte differentiation is a particularly neat example, because two integrin-GFR switches occur. In the first switch, PDGFaR collaborates with the vitronectin receptor avb3 integrin to promote proliferation of oligodendrocytes but, upon contact between cell processes and laminin-2, the same GFR (now in lipid rafts) works with a6b1 integrin to send survival signals. In the second switch, the EGF-family protein neuregulin sends survival and proliferation signals in oligodendrocyte precursors but, upon contact with laminin-2, the neuregulin receptors ErbB2 and ErbB4 provide signals that promote oligodendrocyte differentiation instead6.
Integrins control the cell-division axis: In studies conducted with conventional 2D tissue culture, cells divide in the plane of the dish to which they adhere, requiring alignment of the mitotic spindle parallel to the substratum (in the xy direction). Using micro-patterned ECM substrata that allow single cells to adhere with specific topologies, it has been shown that the tension exerted by the ECM (through integrin-containing adhesion) generates defined actin-cytoskeleton force fields within cells during interphase. During metaphase, cells round up to undergo mitosis, but retraction fibres transfer the polarity of tension that was established in interphase to the astral microtubules (which link the spindle poles with the cell cortex and, therefore, the plasma membrane), thereby aligning the mitotic spindle. Retraction fibres are bound to the substratum by integrins, so cell-matrix adhesion determines the internal architecture of cells, which subsequently defines the spindle position and, therefore, the division axis at mitosis7.
References
1. Michishita, M, Videm, V. and Arnaout, MA (1993). A novel divalentcation binding site in the A domain of the a integrin CR3 (CD11b/CD18) is essential for ligand binding. Cell., 72: 857-867.
2. Lee OJ, Rieu, Arnaout MA and Liddington, R. (1995a). Crystal structure of the A-domain from the b ?subunit of the integrin CR3 (CD11a/CD18). Cell., 80: 631-638.
3. Woodside DG, Liu S and Ginsberg MH (2001). Integrin activation. Thromb. Haemost., 86, 316-323.
4. Velling T, Stefansson A and Johansson S (2008). EGFR and beta1 integrins utilize different signaling pathways to activate Akt. Exp. Cell Res., 314: 309-316.
5. Pullan S, Wilson J, Metcalfe A, Edwards GM, Goberdhan N, Tilly J, Hickman JA, Dive C. and Streuli CH (1996). Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J. Cell Sci., 109: 631- 642.
6. Baron W, Colognato H and ffrench-Constant C (2005). Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia., 49: 467- 479.
7. Thery, M, Racine V, Pepin A, Piel M, Chen Y , Sibarita JB and Bornens M (2005). The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol., 7: 947-953.
| DOI | 名称 | |
|---|---|---|
| 10.1034/j.1399-3011.2003.21040.x | Conformational studies of antimetastatic laminin-1 derived peptides in different solvent systems, using solution NMR spectroscopy | 下载 |
| 10.1016/0006-291x(91)91542-k | Amino acids and peptides. XIV. Laminin related peptides and their inhibitory effect on experimental metastasis formation | 下载 |
| 10.1126/science.2961059 | YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation | 下载 |
| 10.1021/bi00396a004 | A pentapeptide from the laminin B1 chain mediates cell adhesion and binds the 67,000 laminin receptor | 下载 |
多肽H2N-Tyr-Ile-Gly-Ser-Arg-NH2的合成步骤:
1、合成MBHA树脂:取若干克的MBHA树脂(如初始取代度为0.5mmol/g)和1倍树脂摩尔量的Fmoc-Linker-OH加入到反应器中,加入DMF,搅拌使氨基酸完全溶解。再加入树脂2倍量的DIEPA,搅拌混合均匀。再加入树脂0.95倍量的HBTU,搅拌混合均匀。反应3-4小时后,用DMF洗涤3次。用2倍树脂体积的10%乙酸酐/DMF 进行封端30分钟。然后再用DMF洗涤3次,甲醇洗涤2次,DCM洗涤2次,再用甲醇洗涤2次。真空干燥12小时以上,得到干燥的树脂{Fmoc-Linker-MHBA Resin},测定取代度。这里测得取代度为 0.3mmol/g。结构如下图:
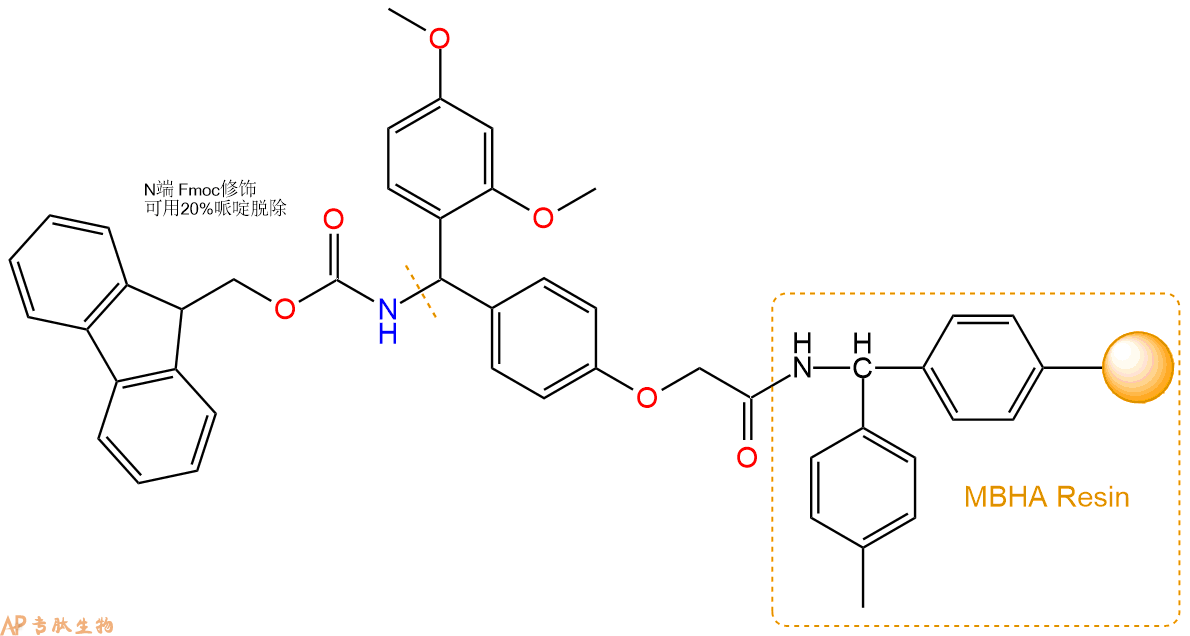
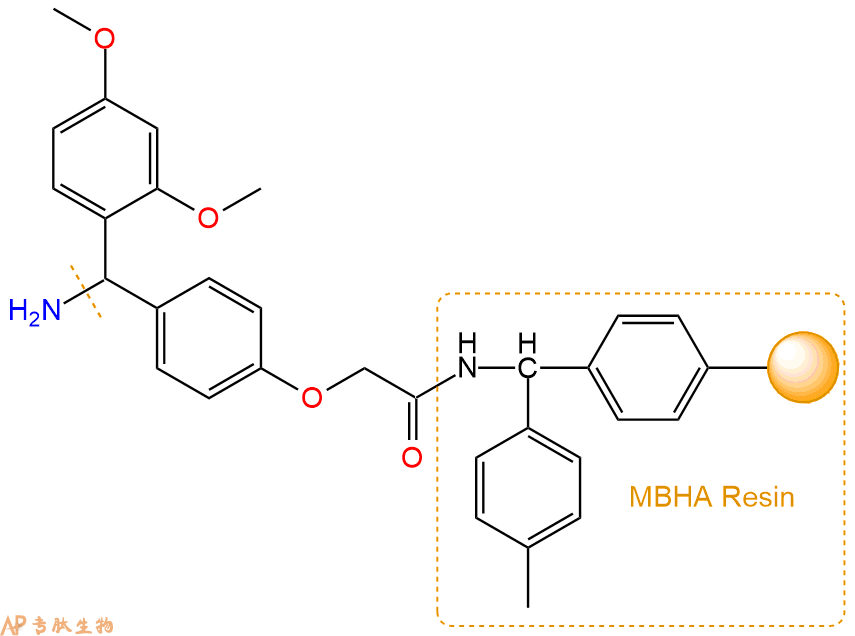
2、脱Fmoc:取1.04g的上述树脂,用DCM或DMF溶胀20分钟。用DMF洗涤2遍。加3倍树脂体积的20%Pip/DMF溶液,鼓氮气30分钟,然后2倍树脂体积的DMF 洗涤5次。得到 H2N-Linker-MBHA Resin 。(此步骤脱除Fmoc基团,茚三酮检测为蓝色,Pip为哌啶)。结构图如下:
3、缩合:取0.94mmol Fmoc-Arg(Pbf)-OH 氨基酸,加入到上述树脂里,加适当DMF溶解氨基酸,再依次加入1.87mmol DIPEA,0.89mmol HBTU。反应30分钟后,取小样洗涤,茚三酮检测为无色。用2倍树脂体积的DMF 洗涤3次树脂。(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。得到Fmoc-Arg(Pbf)-Linker-MBHA Resin。氨基酸:DIPEA:HBTU:树脂=3:6:2.85:1(摩尔比)。结构图如下:

4、依次循环步骤二、步骤三,依次得到
H2N-Arg(Pbf)-Linker-MBHA Resin
Fmoc-Ser(tBu)-Arg(Pbf)-Linker-MBHA Resin
H2N-Ser(tBu)-Arg(Pbf)-Linker-MBHA Resin
Fmoc-Gly-Ser(tBu)-Arg(Pbf)-Linker-MBHA Resin
H2N-Gly-Ser(tBu)-Arg(Pbf)-Linker-MBHA Resin
Fmoc-Ile-Gly-Ser(tBu)-Arg(Pbf)-Linker-MBHA Resin
H2N-Ile-Gly-Ser(tBu)-Arg(Pbf)-Linker-MBHA Resin
Fmoc-Tyr(tBu)-Ile-Gly-Ser(tBu)-Arg(Pbf)-Linker-MBHA Resin
以上中间结构,均可在专肽生物多肽计算器-多肽结构计算器中,一键画出。
最后再经过步骤二得到 H2N-Tyr(tBu)-Ile-Gly-Ser(tBu)-Arg(Pbf)-Linker-MBHA Resin,结构如下:
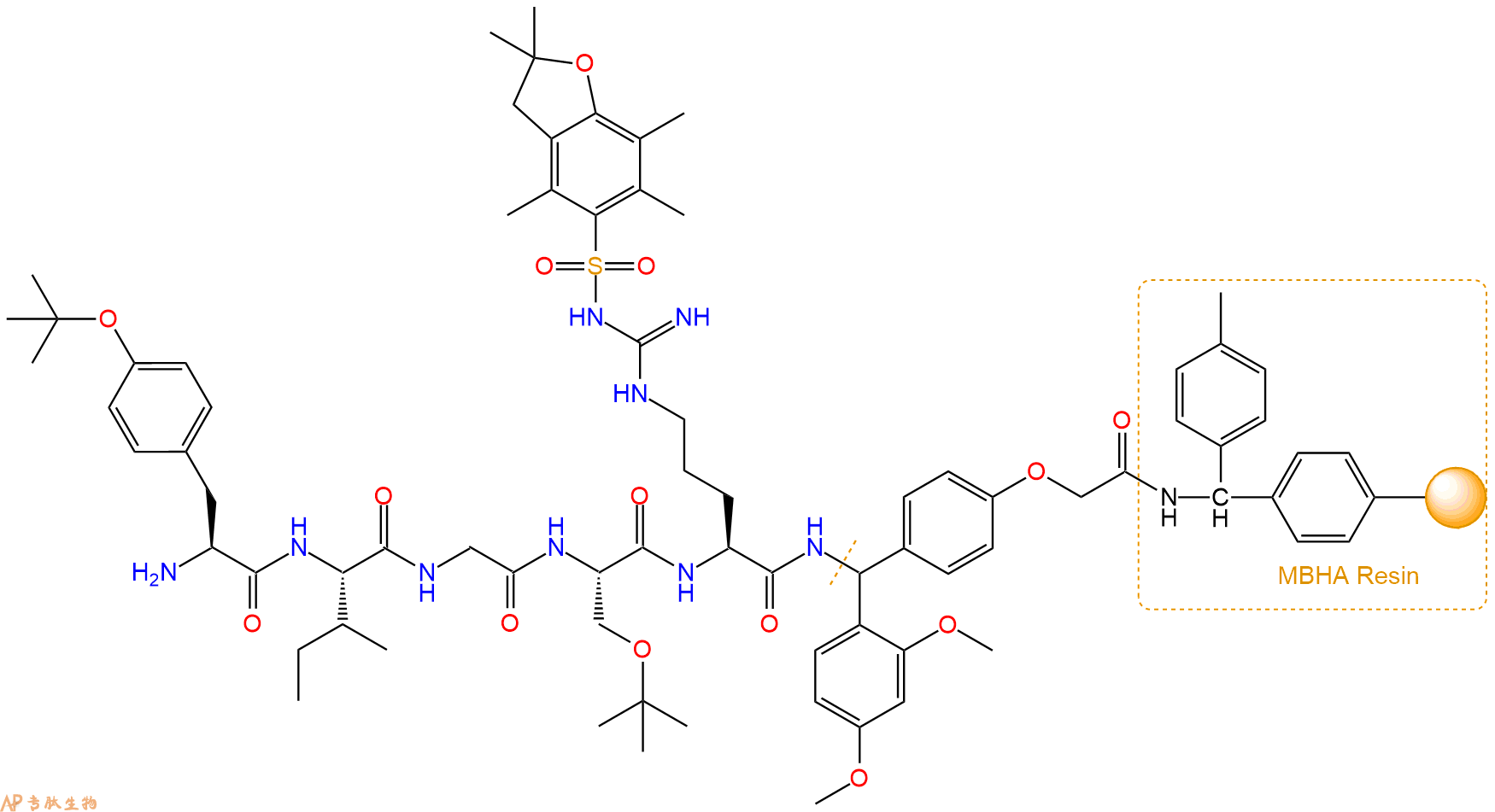
5、切割:6倍树脂体积的切割液(或每1g树脂加8ml左右的切割液),摇床摇晃 2小时,过滤掉树脂,用冰无水乙醚沉淀滤液,并用冰无水乙醚洗涤沉淀物3次,最后将沉淀物放真空干燥釜中,常温干燥24小试,得到粗品H2N-Tyr-Ile-Gly-Ser-Arg-NH2。结构图见产品结构图。
切割液选择:1)TFA:H2O=95%:5%
2)TFA:H2O:TIS=95%:2.5%:2.5%
3)三氟乙酸:茴香硫醚:1,2-乙二硫醇:苯酚:水=87.5%:5%:2.5%:2.5%:2.5%
(前两种适合没有容易氧化的氨基酸,例如Trp、Cys、Met。第三种适合几乎所有的序列。)
6、纯化冻干:使用液相色谱纯化,收集目标峰液体,进行冻干,获得蓬松的粉末状固体多肽。不过这时要取小样复测下纯度 是否目标纯度。
7、最后总结:
杭州专肽生物技术有限公司(ALLPEPTIDE https://www.allpeptide.com)主营定制多肽合成业务,提供各类长肽,短肽,环肽,提供各类修饰肽,如:荧光标记修饰(CY3、CY5、CY5.5、CY7、FAM、FITC、Rhodamine B、TAMRA等),功能基团修饰肽(叠氮、炔基、DBCO、DOTA、NOTA等),同位素标记肽(N15、C13),订书肽(Stapled Peptide),脂肪酸修饰肽(Pal、Myr、Ste),磷酸化修饰肽(P-Ser、P-Thr、P-Tyr),环肽(酰胺键环肽、一对或者多对二硫键环),生物素标记肽,PEG修饰肽,甲基化修饰肽
以上所有内容,为专肽生物原创内容,请勿发布到其他网站上。





