400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研
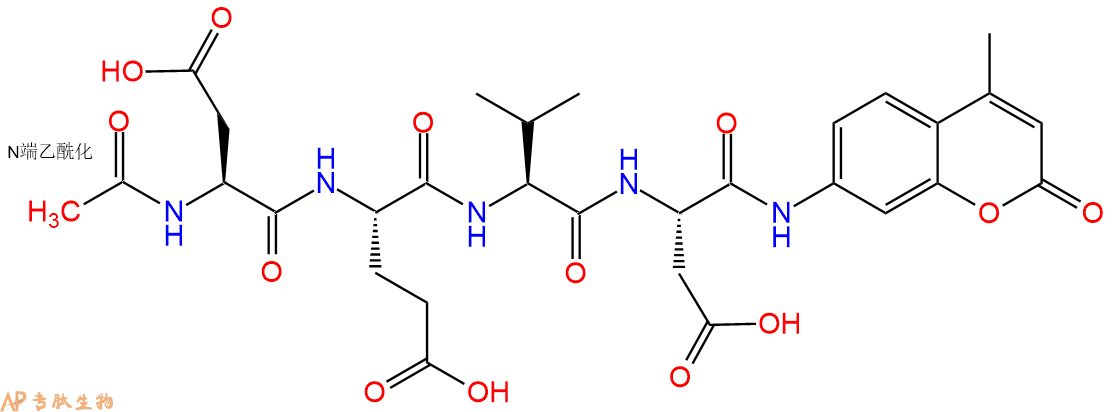
A continuous fluorometric assay employing Ac-DEVD-AMC has been developed for the measurement of caspase-3, a cysteine protease also called apopain, Yama or CPP-32. The design of this substrate Ac-DEVD-AMC was based on the substrate motif that has been use
编号:145638
CAS号:169332-61-0
单字母:Ac-DEVD-AMC
| 编号: | 145638 |
| 中文名称: | Caspase 3 (Apopain) Substrate 1m, fluorogenic |
| 英文名: | Caspase 3 (Apopain) Substrate 1m, fluorogenic |
| 英文同义词: | Apopain Substrate、Caspase 3 (Apopain) Substrate、Caspase 3 Apopain Substrate |
| CAS号: | 169332-61-0 |
| 单字母: | Ac-DEVD-AMC |
| 三字母: | Ac N端乙酰化封端,一种常见的修饰方式,常用于模拟蛋白质中的肽片段。 -AspL-天冬氨酸:aspartic acid。系统命名为(2S)-氨基-丁二酸。是编码氨基酸,又是神经递质。符号:D,Asp。D-天冬氨酸存在于多种细菌的细胞壁和短杆菌肽A中。 -GluL-谷氨酸:glutamic acid。系统命名为(2S)-氨基-戊二酸。是编码氨基酸。符号:E,Glu。D-谷氨酸存在于多种细菌的细胞壁和某些细菌杆菌肽中。 -ValL-缬氨酸:valine。系统命名为(2S)-氨基-3-甲基丁酸。是编码氨基酸。是哺乳动物的必需氨基酸。符号:V,Val。在某些放线菌素如缬霉素中存在 D-缬氨酸。 -AspL-天冬氨酸:aspartic acid。系统命名为(2S)-氨基-丁二酸。是编码氨基酸,又是神经递质。符号:D,Asp。D-天冬氨酸存在于多种细菌的细胞壁和短杆菌肽A中。 -AMC7-氨基-4-甲基香豆素(AMC或Amc)是一种荧光染料,其激发波长为350纳米,发射波长为450纳米。 |
| 氨基酸个数: | 4 |
| 分子式: | C30H37O13N5 |
| 平均分子量: | 675.64 |
| 精确分子量: | 675.24 |
| 等电点(PI): | - |
| pH=7.0时的净电荷数: | -3 |
| 平均亲水性: | 1.875 |
| 疏水性值: | -1.58 |
| 外观与性状: | 白色粉末状固体 |
| 消光系数: | - |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 纯度: | 95%、98% |
| 盐体系: | 可选TFA、HAc、HCl或其它 |
| 生成周期: | 2-3周 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 细胞凋亡肽(Apoptosis Peptides) 半胱氨酸蛋白酶(Caspase) 酶底物肽(Substrate Peptide) AMC修饰肽 |
| 参考文献(References): | D.W. Nicholson, Nature, 376 (1995) J. Xiang et al., Proc. Natl. Acad. Sci. USA, 93, 14559 (1996) |
已经开发了使用Ac-DEVD-AMC的连续荧光测定法来测量胱天蛋白酶-3,一种半胱氨酸蛋白酶,也称为apopain,Yama或CPP-32。该底物Ac-DEVD-AMC的设计基于已成功用于caspase-1的底物基序,使用聚(ADP-核糖)聚合酶切割位点P₁-P₄ 四肽。
A continuous fluorometric assay employing Ac-DEVD-AMC has been developed for the measurement of caspase-3, a cysteine protease also called apopain, Yama or CPP-32. The design of this substrate Ac-DEVD-AMC was based on the substrate motif that has been used successfully with caspase-1, using the poly(ADP-ribose) polymerase cleavage site P₁-P₄ tetrapeptide.
Ac-Tyr-Val-Gly是一种线粒体蛋白,可调节线粒体功能并参与细胞凋亡的调节。Ac-Tyr-Val-Gly与核DNA相互作用并调节转录,翻译和复制。Ac-Tyr-Val-Gly已被证明对肝细胞有毒;然而,它已被证明对神经元死亡或凋亡途径没有影响。这些作用可能是由于其在神经元中诱导蛋白水解活性的能力及其激活促凋亡蛋白如Bax的能力。
Ac-Tyr-Val-Gly is a mitochondrial protein that regulates mitochondrial functions and is involved in the regulation of apoptosis. Ac-Tyr-Val-Gly interacts with nuclear DNA and regulates transcription, translation, and replication. Ac-Tyr-Val-Gly has been shown to be toxic to liver cells; however, it has been shown to have no effect on neuronal death or apoptosis pathway. These effects may be due to its ability to induce proteolytic activity in neurons and its ability to activate proapoptotic proteins such as Bax.
Ac-Asp-Glu-Val-Asp-AMC是一种离子通道激活剂。它与配体的受体位点结合,从而导致蛋白质的构象变化,从而导致离子通道的开放。Ac-Asp-Glu-Val-Asp-AMC是一种分子量为927.6的肽,可溶于水。该产品已被证明在各种条件下稳定,可用作研究工具或药理剂。Ac-Asp-Glu-Val-Asp-AMC也是一种与人CD8蛋白表位结合的抗体,可用于细胞生物学研究,例如激活和抑制这些蛋白。
Ac-Asp-Glu-Val-Asp-AMC is an ion channel activator. It binds to the receptor site of the ligand, which causes a conformational change in the protein that leads to the opening of the ion channel. Ac-Asp-Glu-Val-Asp-AMC is a peptide with a molecular weight of 927.6 and is soluble in water. This product has been shown to be stable under various conditions and can be used as research tool or pharmacological agent. Ac-Asp-Glu-Val-Asp-AMC is also an antibody that binds to an epitope on human CD8 protein and can be used for cell biology studies, such as activation and inhibition of these proteins.
Ac-Asp-Glu-Val-Asp-AMC铵盐是一种小分子,用作体外研究细胞凋亡的工具。Ac-Asp-Glu-Val-Asp-AMC铵盐通过阻断线粒体膜电位并诱导细胞色素c从线粒体释放到细胞质中来诱导细胞凋亡。该药物还诱导胱天蛋白酶3的激活,其启动导致细胞死亡的级联事件。已显示Ac-Asp-Glu-Val-Asp-AMC铵盐对hl60细胞具有抗血管生成作用。这种作用可能是由于其抑制survivin表达的能力,survivin是一种保护细胞免于凋亡的蛋白质。该药物在实验模型中的功效已被证明依赖于toll样受体(TLR)信号通路和线粒体功能。
Ac-Asp-Glu-Val-Asp-AMC ammonium salt is a small molecule that is used as a tool to study apoptosis in vitro. Ac-Asp-Glu-Val-Asp-AMC ammonium salt induces apoptosis by blocking the mitochondrial membrane potential and inducing the release of cytochrome c from mitochondria into the cytoplasm. This drug also induces activation of caspase 3, which initiates the cascade of events leading to cell death. Ac-Asp-Glu-Val-Asp-AMC ammonium salt has been shown to have an antiangiogenic effect on hl60 cells. This effect may be due to its ability to inhibit expression of survivin, a protein that protects cells from apoptosis. The efficacy of this drug in an experimental model has been shown to be dependent on toll like receptor (TLR) signaling pathways and mitochondrial function.
使用聚(ADP-核糖)聚合酶切割位点P1-P4四肽,基于已成功用于caspase-1的四肽AMC基序设计底物。
Designed substrate based on the tetrapeptide-AMC motif that has been used successfully with caspase-1, using the poly (ADP ribose) polymerase cleavage site P1-P4 tetrapeptide.
Definition
Apoptosis or programmed cell death is a normal component of the development and health of multicellular organisms. Cells die in response to a variety of stimuli and during apoptosis they do so in a controlled, regulated fashion.
Discovery
In 1885, Flemming W described the process of programmed cell death. John Kerr's discovery, in late 1960s, initially called "shrinkage necrosis" but which he later renamed "apoptosis", came about when his attention was caught by a curious form of liver cell death during his studies of acute liver injury in rats 1,2. Kerr in 1972 proposed the term apoptosis is for mechanism of controlled cell deletion, which appears to play a complementary but opposite role to mitosis in the regulation of animal cell populations. Its morphological features suggest that it is an active, inherently programmed phenomenon, and it has been shown that it can be initiated or inhibited by a variety of environmental stimuli, both physiological and pathological 3.
Structural Characteristics
Heterodimerization between members of the Bcl-2 family of proteins is a key event in the regulation of programmed cell death. The molecular basis for heterodimer formation was investigated by determination of the solution structure of a complex between the survival protein Bcl-xL and the death-promoting region of the Bcl-2-related protein Bak. The structure and binding affinities of mutant Bak peptides indicate that the Bak peptide adopts an amphipathic helix that interacts with Bcl-xL through hydrophobic and electrostatic interactions. Mutations in full-length Bak that disrupt either type of interaction inhibit the ability of Bak to heterodimerize with Bcl-xL 4.
The structure of the 16–amino acid peptide complexed with a biologically active deletion mutant of Bcl-xL was determined by nuclear magnetic resonance spectroscopy (NMR). The structure was determined from a total of 2813 NMR-derived restraints and is well defined by the NMR data. The Bak peptide forms a helix when complexed to Bcl-xL. The COOH terminal portion of the Bak peptide interacts predominantly with residues in the BH2 and BH3 regions. Melanoma inhibitor of apoptosis (ML-IAP) is a potent anti-apoptotic protein that is upregulated in a number of melanoma cell lines but not expressed in most normal adult tissues. Overexpression of IAP proteins, such as ML-IAP or the ubiquitously expressed X-chromosome-linked IAP (XIAP), in human cancers has been shown to suppress apoptosis induced by a variety of stimuli. X-ray crystal structures of ML-IAP-BIR in complex with Smac- and phage-derived peptides, together with peptide structure−activity-relationship data, indicate that the peptides can be modified to provide increased binding affinity and selectivity for ML-IAP-BIR relative to XIAP-BIR3 5.
Mode of Action
Upon receiving specific signals instructing the cells to undergo apoptosis a number of distinctive changes occur in the cell. Families of proteins known as caspases are typically activated in the early stages of apoptosis. These proteins breakdown or cleave key cellular components that are required for normal cellular function including structural proteins in the cytoskeleton and nuclear proteins such as DNA repair enzymes. The caspases can also activate other degradative enzymes such as DNases, which begin to cleave the DNA in the nucleus.
Apoptotic cells display distinctive morphology during the apoptotic process. Typically, the cell begins to shrink following the cleavage of lamins and actin filaments in the cytoskeleton. The breakdown of chromatin in the nucleus often leads to nuclear condensation and in many cases the nuclei of apoptotic cells take on a "horse-shoe" like appearance. Cells continue to shrink, packaging themselves into a form that allows for their removal by macrophages. There are a number of mechanisms through which apoptosis can be induced in cells. The sensitivity of cells to any of these stimuli can vary depending on a number of factors such as the expression of pro- and anti-apoptotic proteins (eg. the Bcl-2 proteins or the Inhibitor of Apoptosis Proteins), the severity of the stimulus and the stage of the cell cycle. The Bcl-2 family of proteins plays a central role in the regulation of apoptotic cell death induced by a wide variety of stimuli. Some proteins within this family, including Bcl-2 and Bcl-xL, inhibit programmed cell death, and others, such as Bax and Bak, can promote apoptosis 6, 7.
Functions
For development, Apoptosis is as needed for proper development as mitosis is. Examples: The resorption of the tadpole tail at the time of its metamorphosis into a frog occurs by apoptosis.
Integrity of the organism, Apoptosis is needed to destroy cells that represent a threat to the integrity of the organism. Examples: Cells infected with viruses8.
Cells of the immune system, as cell-mediated immune responses wane, the effector cells must be removed to prevent them from attacking body constituents. CTLs induce apoptosis in each other and even in themselves 9.
Cells with DNA damage, damage to its genome can cause a cell to disrupt proper embryonic development leading to birth defects to become cancerous.
References
1. Kerr JF (1965). A histochemical study of hypertrophy and ischaemic injury of rat liver with special reference to changes in lysosomes. Journal of Pathology and Bacteriology, 90(90):419-435.
2. Kerr JF, Wyllie AH, Currie AR (1972). Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer., 26(4):239-257.
3. O'Rourke MG, Ellem KA (2000). John Kerr and apoptosis. Med. J. Aust., 173(11-12): 616-617.
4. Franklin MC, Kadkhodayan S, Ackerly H, Alexandru D, Distefano MD, Elliott LO, Flygare JA, Mausisa G, Okawa DC, Ong D, Vucic D, Deshayes K, Fairbrother WJ (2003). Structure and function analysis of peptide antagonists of melanoma inhibitor of apoptosis (ML-IAP). Biochemistry, 42(27):8223-8231.
5. Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW (1997). Structure of bcl-xl-bak peptide complex: recognition between regulators of apoptosis. Science, 275(5302):983-986.
6. Hanada M, Aimé-Sempé C, Sato T, Reed JC (1995). Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J. Biol. Chem., 270(20):11962-11969.
7. Cheng EHY, Levine B, Boise LH, Thompson CB, Hardwic JM (1996). Bax-independent inhibition of apoptosis by Bcl-xL.Nature, 379:554-556.
8. Alimonti JB, Ball TB, Fowke KR (2003). Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J Gen Virology., 84(84): 1649-1661.
9. Werlen G, Hausmann B, Naeher D, Palmer E (2003). Signaling life and death in the thymus: timing is everything. Science. 299(5614):1859-1863.
Definition
Caspases are a family of aspartate specific cysteine proteases that play an important role in apoptosis, necrosis and inflammation1.
Discovery
Caspases were first identified in the nematode C. elegans. It was found that the gene ced-3 was required for cell death during C.elegans development2. In 1993, the protein encoded by the ced-3 gene was identified as a cysteine protease and it was found that it had similar properties to the mammalian interleukin-1-beta converting enzyme (ICE) (now known as caspase 1) which at the time was the only known caspase3. Other mammalian caspases were subsequently identified.
Classification
There are three types of apoptotic caspases: initiator, effector and inflammatory caspases. Initiator caspases (e.g. CASP2, CASP8, CASP9 and CASP10) cleave inactive pro-forms of effector caspases, thereby activating them4. Effector caspases (e.g. CASP3, CASP6 and CASP7) in turn cleave other protein substrates within the cell, to trigger the apoptotic process4. Inflammatory caspases are involved in immune response (e.g. CASP1, CASP4, CASP5, CASP11, CASP12 and CASP13). Caspase inhibitors regulate the initiation of this cascade4.
Structural Characteristics
Caspases are synthesized as inactive zymogens or procaspases. Activation of caspases occurs by cleavage of the prodomain in the procaspases5. The caspase catalytic domain is composed of a twisted, mostly parallel ß-sheet sandwiched between two layers of a-helices. Also they contain an active cysteine residue in their catalytic domain5. In addition to the catalytic domain, both inflammatory and initiator caspases carry at their N-termini, one or two copies of CARD or DED modules, which are critical for their activation in vivo. These modules are mainly composed of six antiparallel a-helices, with helices a1–a5 building an a-helical Greek key5. The general structure of a caspase inhibitor is [tetrapeptide]-CO-CH2-X, that binds to the Cys285 in the active site of caspases5.
Mode of action
Caspases cleave the substrate after an Asp residue6. There are several hundred substrates for caspases. Initially activation of initiator caspases occurs as a result of an extrinsic or intrinsic death signal6. Activated initiator caspases cleave effector caspases that in turn cleave the substrate at an Asp residue6. For example, caspase-8 cleaves the pro-apoptotic protein Bid that gets activated and translocates into the mitochondria where it activates other pro-apoptotic proteins, Bax and Bak thus amplifying the death signal6.
Functions
Caspases such as caspase-1 are involved in the activation of pro-inflammatory cytokines such as Interleukin 1 and interleukin 185,6. Caspases play an important role in apoptosis. One of the hallmark feature of apoptotic cell death is genomic disassembly and proteolysis5,6. By cleaving their substartes, caspases inactivate cell cycle progression and DNA repair processes. They also activate several pro-apoptotic proteins5,6. In some cases Caspases’ role in aberrant processing events has shown their involvement in neurodegenerative disorders such as Huntington disease and Alzheimer’s disease6. Some of the final targets of caspases include: nuclear lamins, ICAD/DFF45 (inhibitor of caspase activated DNase or DNA fragmentation factor 45), PARP (poly-ADP ribose polymerase) and PAK2 (P 21-activated kinase 2)6. Caspases are also implicated in embryonic development and T and B cell differentiation7.
References
1. Book: Cells by Benjamin L, Lynne C, Vishwanath RL, George P (207), 536-540.
2. Ellis HM, Horvitz HR (1986). Genetic control of programmed cell death in the nematode C. elegans. Cell, 44(6), 817-29.
3. Yuan J, Shaham S, Ledoux S, Ellis HM and Horvitz HR (1993). The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell 75: 641–652.
4. Salvesen GS, Riedl SJ (2008). Caspase mechanisms. Adv Exp Med Biol., 615, 13-23.
5. Prior PF and Salvesen GS (2004). The protein structures that shape caspase activity, specificity,activation and inhibition. Biochem. J., 384, 201–232.
6. Nicholson DW (1999). Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death and Differentiation, 6, 1028 ± 1042.
7. Maelfait J, Beyaert R (2008). Non-apoptotic functions of caspase-8. Biochem Pharmacol., 76(11), 1365-73.
Caspase酶对应的底物,Caspases(半胱氨酸天冬氨酸蛋白酶,半胱氨酸依赖性天冬氨酸定向蛋白酶)是一类蛋白酶家族,其功能与凋亡(程序性细胞死亡),坏死和发烧(炎症)的过程密切相关。
什么是胱天蛋白酶?
胱天蛋白酶(Caspases)是含半胱氨酸的天冬氨酸蛋白水解酶,它们是为细胞凋亡的主要介质。多种受体,例如TNF-α 受体,FasL受体,TLR和死亡受体,以及Bcl-2和凋亡抑制剂(IAP)蛋白家族参与并调节该caspase依赖性凋亡途径。一旦Caspase受到上游信号(外部或内在)刺激被激活,即会参与执行下游蛋白底物的水解作用,并触发一系列事件,导致细胞分解,死亡,吞噬作用和细胞碎片的清除。
人Caspases酶
人的Caspases家族基于序列相似性和生物学功能等共性主要可分为三大类:第一类由具有长胱天蛋白酶募集结构域的“炎症”胱天蛋白酶组成,他们对P4位上的较大的芳香族或疏水性残基具有亲和力。第二类由具有短的前体结构域的“细胞凋亡效应”胱天蛋白酶组成,而第三类由具有长的前提结构域的Pap位置具有亮氨酸或缬氨酸底物亲和力的“凋亡引发剂”胱天蛋白酶组成(表1)。
表1. 人胱天蛋白酶的功能分类:
| 细胞死亡途径 | 半胱天冬酶类型 | 酵素 | 物种 |
| 细胞凋亡 | 启动器 | Caspases 2 | 人与鼠 |
| 细胞凋亡 | 启动器 | Caspases 8 | 人与鼠 |
| 细胞凋亡 | 启动器 | Caspases 9 | 人与鼠 |
| 细胞凋亡 | 启动器 | Caspases 10 | 人的 |
| 细胞凋亡 | 效应器 | Caspases 3 | 人与鼠 |
| 细胞凋亡 | 效应器 | Caspases 6 | 人与鼠 |
| 细胞凋亡 | 效应器 | Caspases 6 | 人与鼠 |
| 细胞焦亡 | 炎性的 | Caspases 1 | 人与鼠 |
| 细胞焦亡 | 炎性的 | Caspases 4 | 人的 |
| 细胞焦亡 | 炎性的 | Caspases 5 | 人的 |
启动器Caspase和效应器Caspase酶
根据其在凋亡胱天蛋白酶途径中的作用,胱天蛋白酶可分为两类:启动器和效应器Caspase酶。启动器和效应器Caspas酶都具有由小亚基和大亚基组成的催化位点,Caspase酶的识别位
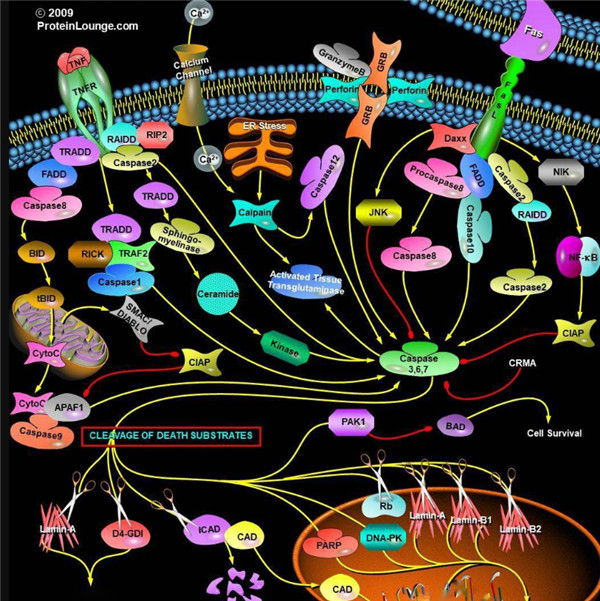
凋亡启动器Caspase酶,例如caspase-2,-8,-9和-10可以启动caspase激活级联反应。Caspase-8对于形成死亡诱导信号复合物(DISC)是必不可少的,并且在激活后,Caspase-8激活下游效应子Caspase(例如Caspase 3)并介导线粒体中细胞色素c的释放。Caspase-8已被证明对IETD肽序列具有相对较高的底物选择性。凋亡效应胱天蛋白酶例如Caspase-3,-6和-7虽然不负责启动级联途径,但是当被激活时,它们在级联的中间和后续步骤中起着不可或缺的作用。Caspase-3(CPP32 / apopain)是关键效应器,因为它放大了来自启动器Caspase的信号,使用对Caspase-3有选择性的DEVD肽序列对活化的Caspase-3进行检测,可以检测Caspase-3的活性。
Caspase酶底物和抑制剂
Caspase底物和抑制剂由两个关键成分组成:Caspase识别序列和信号产生或蛋白酶抑制基序。不同Caspase识别序列不同,一般由三个或四个氨基酸组成(表2)。Caspase酶识别序列的N端通常有乙酰基(Ac)或碳苯甲氧基(Z)基团修饰,以增强膜的通透性。对应的Caspase识别特定的肽序列为其酶促反应切割位点,释放产生信号或抑制信号的基序。Caspase的显色和荧光底物均以相似的方式起作用,其中底物的信号或颜色强度与蛋白水解活性成正比。
表2. Caspase的底物及其序列
| 多肽 | 氨基酸序列 | 对应的Caspase的种类 |
| IETD | Ile-Glu-Thr-Asp | Caspase 8,颗粒酶B |
| DEVD | Asp-Glu-Val-Asp | Caspase 3、6、7、8或10 |
| LEHD | Leu-Glu-His-Asp | Caspase 9 |
| VAD | Val-Ala-Asp | Caspase 1、2、3、6、8、9或10 |
Caspase酶的显色底物
Caspase的显色底物是有Caspase识别序列及生色基团组成,常见的生色团有pNA(对硝基苯胺或4-硝基苯胺),可使用酶标仪或分光光度计在405 nm处进行光密度检测。
表3. Caspase的显色底物
| 底物 | Caspase | 吸收(nm) | 颜色 |
| Ac-DEVD-pNA * CAS 189950-66-1 * | 半胱天冬酶3 | 405 nm | 黄色 |
| Z-DEVD-pNA | 半胱天冬酶3 | 405 nm | 黄色 |
| Z-IETD-pNA * CAS 219138-21-3 * | 半胱天冬酶8,颗粒酶B | 405 nm | 黄色 |
Caspase的荧光底物
Caspase的荧光底物的结构包含与半胱天冬酶识别相关的荧光团,例如7-氨基-4-甲基香豆素(AMC),7-氨基-4-三氟甲基香豆素(AFC), Rhodamine 110(R110)或ProRed™620。R110的Caspase底物比基于香豆素的Caspase底物(例如AMC和AFC)更敏感,但由于两步裂解过程,其动态范围更窄。 建议将R110标记的Caspase底物用于终点法测定,而将AMC和AFC标记的 Caspase底物用于动力学测定。
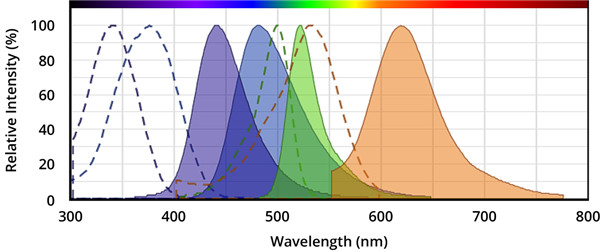
图.从左到右,分别是AMC(7-氨基-4-甲基香豆素),AFC(7-氨基-4-三氟甲基香豆素),Rhodamine 110(R110)和ProRed™620的激发和发射光谱。
表4.荧光半胱天冬酶底物。
| 底物名称 | 对应的Caspase | Ex(nm) | Em(nm) | ε¹ | Φ² |
| Ac-DEVD-AFC * CAS 201608-14-2 * | 半胱天冬酶3、7 | 376 | 482 | 17000 | 0.53 |
| Ac-DEVD-AMC * CAS 169332-61-0 * | 半胱天冬酶3、7 | 341 | 441 | 19000 | N / D |
| Z-DEVD-AFC | 半胱天冬酶3、7 | 376 | 482 | 17000 | 0.53 |
| Z-DEVD-AMC * CAS 1135416-11-3 * | 半胱天冬酶3、7 | 341 | 441 | 19000 | N / D |
| Z-DEVD-ProRed™620 | 半胱天冬酶3、7 | 532 | 619 | N / D | N / D |
| (Z-DEVD)2 -R110 * CAS 223538-61-2 * | 半胱天冬酶3、7 | 500 | 522 | 80000 | N / D |
| Z-DEVD-ProRed™620 | 半胱天冬酶3、7 | 532 | 619 | N / D | N / D |
| Ac-IETD-AFC * CAS 211990-57-7 * | 半胱天冬酶8,颗粒酶B | 376 | 482 | 17000 | 0.53 |
| Z-IETD-AFC * CAS 219138-02-0 * | 半胱天冬酶8,颗粒酶B | 376 | 482 | 17000 | 0.53 |
注意:
1.ε=在其最大吸收波长处的摩尔消光系数(单位= cm -1M -1)。
2.Φ=水性缓冲液(pH 7.2)中的荧光量子产率。
Caspase抑制剂
Caspase抑制剂能与Caspase的活性位点结合并形成可逆或不可逆的连接,通常,Caspase抑制剂的结构由Caspase识别序列,诸如醛(-CHO)或氟甲基酮(-FMK)的官能团组成。具有醛官能团的胱天蛋白酶抑制剂是可逆的,而具有FMK的抑制剂是不可逆的。半胱天冬酶底物和抑制剂都具有较小的细胞毒性作用,因此,它们是研究半胱天冬酶活性的有用工具。
表5. 可逆和不可逆的Caspase酶抑制剂
| 抑制剂 | Caspase的种类 | 是否可逆 | Ex(nm) | Em(nm) |
| Ac-DEVD-CHO * CAS 169332-60-9 * | 半胱天冬酶3、7 | 可逆的 | -- | -- |
| Ac-IETD-CHO * CAS 191338-86-0 * | 半胱天冬酶8 | 可逆的 | -- | -- |
| mFluor™450-VAD-FMK | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 406 | 445 |
| mFluor™510-VAD-FMK | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 412 | 505 |
| FITC-C6-DEVD-FMK | 半胱天冬酶3、7 | 不可逆的 | 491 | 516 |
| FITC-C6-DEVD-FMK | 半胱天冬酶3、7 | 不可逆的 | 491 | 516 |
| FITC-C6-LEHD-FMK | 半胱天冬酶9 | 不可逆的 | 491 | 516 |
| FITC-C6-LEHD-FMK | 半胱天冬酶9 | 不可逆的 | 491 | 516 |
| FAM-VAD-FMK | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 493 | 517 |
| SRB-VAD-FMK [磺胺丁胺B-VAD-FMK] | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 559 | 577 |
Caspase酶对应的底物,Caspases(半胱氨酸天冬氨酸蛋白酶,半胱氨酸依赖性天冬氨酸定向蛋白酶)是一类蛋白酶家族,其功能与凋亡(程序性细胞死亡),坏死和发烧(炎症)的过程密切相关。

什么是胱天蛋白酶?
胱天蛋白酶(Caspases)是含半胱氨酸的天冬氨酸蛋白水解酶,它们是为细胞凋亡的主要介质。多种受体,例如TNF-α 受体,FasL受体,TLR和死亡受体,以及Bcl-2和凋亡抑制剂(IAP)蛋白家族参与并调节该caspase依赖性凋亡途径。一旦Caspase受到上游信号(外部或内在)刺激被激活,即会参与执行下游蛋白底物的水解作用,并触发一系列事件,导致细胞分解,死亡,吞噬作用和细胞碎片的清除。
人Caspases酶
人的Caspases家族基于序列相似性和生物学功能等共性主要可分为三大类:第一类由具有长胱天蛋白酶募集结构域的“炎症”胱天蛋白酶组成,他们对P4位上的较大的芳香族或疏水性残基具有亲和力。第二类由具有短的前体结构域的“细胞凋亡效应”胱天蛋白酶组成,而第三类由具有长的前提结构域的Pap位置具有亮氨酸或缬氨酸底物亲和力的“凋亡引发剂”胱天蛋白酶组成(表1)。
表1. 人胱天蛋白酶的功能分类:
| 细胞死亡途径 | 半胱天冬酶类型 | 酵素 | 物种 |
| 细胞凋亡 | 启动器 | Caspases 2 | 人与鼠 |
| 细胞凋亡 | 启动器 | Caspases 8 | 人与鼠 |
| 细胞凋亡 | 启动器 | Caspases 9 | 人与鼠 |
| 细胞凋亡 | 启动器 | Caspases 10 | 人的 |
| 细胞凋亡 | 效应器 | Caspases 3 | 人与鼠 |
| 细胞凋亡 | 效应器 | Caspases 6 | 人与鼠 |
| 细胞凋亡 | 效应器 | Caspases 6 | 人与鼠 |
| 细胞焦亡 | 炎性的 | Caspases 1 | 人与鼠 |
| 细胞焦亡 | 炎性的 | Caspases 4 | 人的 |
| 细胞焦亡 | 炎性的 | Caspases 5 | 人的 |
启动器Caspase和效应器Caspase酶
根据其在凋亡胱天蛋白酶途径中的作用,胱天蛋白酶可分为两类:启动器和效应器Caspase酶。启动器和效应器Caspas酶都具有由小亚基和大亚基组成的催化位点,Caspase酶的识别位

凋亡启动器Caspase酶,例如caspase-2,-8,-9和-10可以启动caspase激活级联反应。Caspase-8对于形成死亡诱导信号复合物(DISC)是必不可少的,并且在激活后,Caspase-8激活下游效应子Caspase(例如Caspase 3)并介导线粒体中细胞色素c的释放。Caspase-8已被证明对IETD肽序列具有相对较高的底物选择性。凋亡效应胱天蛋白酶例如Caspase-3,-6和-7虽然不负责启动级联途径,但是当被激活时,它们在级联的中间和后续步骤中起着不可或缺的作用。Caspase-3(CPP32 / apopain)是关键效应器,因为它放大了来自启动器Caspase的信号,使用对Caspase-3有选择性的DEVD肽序列对活化的Caspase-3进行检测,可以检测Caspase-3的活性。
Caspase酶底物和抑制剂
Caspase底物和抑制剂由两个关键成分组成:Caspase识别序列和信号产生或蛋白酶抑制基序。不同Caspase识别序列不同,一般由三个或四个氨基酸组成(表2)。Caspase酶识别序列的N端通常有乙酰基(Ac)或碳苯甲氧基(Z)基团修饰,以增强膜的通透性。对应的Caspase识别特定的肽序列为其酶促反应切割位点,释放产生信号或抑制信号的基序。Caspase的显色和荧光底物均以相似的方式起作用,其中底物的信号或颜色强度与蛋白水解活性成正比。
表2. Caspase的底物及其序列
| 多肽 | 氨基酸序列 | 对应的Caspase的种类 |
| IETD | Ile-Glu-Thr-Asp | Caspase 8,颗粒酶B |
| DEVD | Asp-Glu-Val-Asp | Caspase 3、6、7、8或10 |
| LEHD | Leu-Glu-His-Asp | Caspase 9 |
| VAD | Val-Ala-Asp | Caspase 1、2、3、6、8、9或10 |
Caspase酶的显色底物
Caspase的显色底物是有Caspase识别序列及生色基团组成,常见的生色团有pNA(对硝基苯胺或4-硝基苯胺),可使用酶标仪或分光光度计在405 nm处进行光密度检测。
表3. Caspase的显色底物
| 底物 | Caspase | 吸收(nm) | 颜色 |
| Ac-DEVD-pNA * CAS 189950-66-1 * | 半胱天冬酶3 | 405 nm | 黄色 |
| Z-DEVD-pNA | 半胱天冬酶3 | 405 nm | 黄色 |
| Z-IETD-pNA * CAS 219138-21-3 * | 半胱天冬酶8,颗粒酶B | 405 nm | 黄色 |
Caspase的荧光底物
Caspase的荧光底物的结构包含与半胱天冬酶识别相关的荧光团,例如7-氨基-4-甲基香豆素(AMC),7-氨基-4-三氟甲基香豆素(AFC), Rhodamine 110(R110)或ProRed™620。R110的Caspase底物比基于香豆素的Caspase底物(例如AMC和AFC)更敏感,但由于两步裂解过程,其动态范围更窄。 建议将R110标记的Caspase底物用于终点法测定,而将AMC和AFC标记的 Caspase底物用于动力学测定。

图.从左到右,分别是AMC(7-氨基-4-甲基香豆素),AFC(7-氨基-4-三氟甲基香豆素),Rhodamine 110(R110)和ProRed™620的激发和发射光谱。
表4.荧光半胱天冬酶底物。
| 底物名称 | 对应的Caspase | Ex(nm) | Em(nm) | ε¹ | Φ² |
| Ac-DEVD-AFC * CAS 201608-14-2 * | 半胱天冬酶3、7 | 376 | 482 | 17000 | 0.53 |
| Ac-DEVD-AMC * CAS 169332-61-0 * | 半胱天冬酶3、7 | 341 | 441 | 19000 | N / D |
| Z-DEVD-AFC | 半胱天冬酶3、7 | 376 | 482 | 17000 | 0.53 |
| Z-DEVD-AMC * CAS 1135416-11-3 * | 半胱天冬酶3、7 | 341 | 441 | 19000 | N / D |
| Z-DEVD-ProRed™620 | 半胱天冬酶3、7 | 532 | 619 | N / D | N / D |
| (Z-DEVD)2 -R110 * CAS 223538-61-2 * | 半胱天冬酶3、7 | 500 | 522 | 80000 | N / D |
| Z-DEVD-ProRed™620 | 半胱天冬酶3、7 | 532 | 619 | N / D | N / D |
| Ac-IETD-AFC * CAS 211990-57-7 * | 半胱天冬酶8,颗粒酶B | 376 | 482 | 17000 | 0.53 |
| Z-IETD-AFC * CAS 219138-02-0 * | 半胱天冬酶8,颗粒酶B | 376 | 482 | 17000 | 0.53 |
注意:
1.ε=在其最大吸收波长处的摩尔消光系数(单位= cm -1M -1)。
2.Φ=水性缓冲液(pH 7.2)中的荧光量子产率。
Caspase抑制剂
Caspase抑制剂能与Caspase的活性位点结合并形成可逆或不可逆的连接,通常,Caspase抑制剂的结构由Caspase识别序列,诸如醛(-CHO)或氟甲基酮(-FMK)的官能团组成。具有醛官能团的胱天蛋白酶抑制剂是可逆的,而具有FMK的抑制剂是不可逆的。半胱天冬酶底物和抑制剂都具有较小的细胞毒性作用,因此,它们是研究半胱天冬酶活性的有用工具。
表5. 可逆和不可逆的Caspase酶抑制剂
| 抑制剂 | Caspase的种类 | 是否可逆 | Ex(nm) | Em(nm) |
| Ac-DEVD-CHO * CAS 169332-60-9 * | 半胱天冬酶3、7 | 可逆的 | -- | -- |
| Ac-IETD-CHO * CAS 191338-86-0 * | 半胱天冬酶8 | 可逆的 | -- | -- |
| mFluor™450-VAD-FMK | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 406 | 445 |
| mFluor™510-VAD-FMK | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 412 | 505 |
| FITC-C6-DEVD-FMK | 半胱天冬酶3、7 | 不可逆的 | 491 | 516 |
| FITC-C6-DEVD-FMK | 半胱天冬酶3、7 | 不可逆的 | 491 | 516 |
| FITC-C6-LEHD-FMK | 半胱天冬酶9 | 不可逆的 | 491 | 516 |
| FITC-C6-LEHD-FMK | 半胱天冬酶9 | 不可逆的 | 491 | 516 |
| FAM-VAD-FMK | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 493 | 517 |
| SRB-VAD-FMK [磺胺丁胺B-VAD-FMK] | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 559 | 577 |
多肽AMC标记,全称多肽7-氨基-4-甲基香豆素标记(Peptide 7-Amino-4-Methylcoumarin Labeling),是一种将荧光分子“AMC”通过特异性共价键连接到多肽特定位点的修饰技术。其核心价值在于为多肽赋予可追踪、可定量的荧光特性,使其成为兼具生物活性与检测功能的“荧光探针”,广泛应用于生物医学科研、酶学分析及药物筛选等领域。
AMC(7-氨基-4-甲基香豆素)是该标记技术的核心荧光基团,其独特的化学与光学特性决定了标记效果,核心优势包括:
光学性能稳定:激发波长约340-360 nm,发射波长约440-460 nm,荧光量子产率高,光漂白抗性强,且背景荧光低,能有效提升检测灵敏度;
反应活性适配:AMC常以“活性酯衍生物”形式(如AMC-NHS酯、AMC-COOH)存在,可与多肽分子末端(N端/C端)或侧链(如赖氨酸的ε-氨基、天冬氨酸/谷氨酸的羧基)发生酰胺化反应,形成稳定的共价键,且对多肽的空间结构破坏极小;
水溶性适配:AMC分子兼具一定的亲水性与疏水性,标记后不会显著改变多肽的溶解特性,适配后续水性体系的生物实验。
多肽AMC标记的核心是“特异性共价偶联”,需在温和条件下进行以保障多肽生物活性,典型流程如下:
多肽预处理:先通过高效液相色谱(HPLC)等技术纯化目标多肽,去除杂质;同时确认多肽的活性位点(如受体结合位点、酶切位点),避开这些位点选择标记位点(常用N端氨基或C端羧基);
AMC活性酯制备:将AMC与活化试剂(如NHS、DCC)反应,生成高活性的AMC-NHS酯(减少AMC自身聚合,提升与多肽的反应效率);
偶联反应:在缓冲体系(如PBS缓冲液,pH 7.0-8.0)中混合多肽与AMC活性酯,控制反应温度(25℃或4℃)与时间(2-4小时),让AMC活性酯的活性基团与多肽的氨基/羧基发生酰胺化反应,形成稳定的标记产物;
纯化与验证:通过HPLC分离未反应的游离AMC、活化试剂及副产物,收集高纯度的AMC标记多肽;再通过质谱(确认分子量是否符合标记后理论值)、荧光光谱(验证荧光信号强度)进行质控,确保标记成功且多肽活性未受影响。
AMC标记的核心优势的是“标记后多肽保留生物活性,荧光信号可实时定量检测”,因此主要应用于以下场景:
蛋白酶活性检测(最核心应用):将AMC标记在多肽底物的蛋白酶特异性识别序列末端,当蛋白酶切割多肽时,AMC基团被释放(游离AMC的荧光强度远高于结合态),通过检测荧光强度的变化速率,可定量分析蛋白酶的活性(如基质金属蛋白酶MMPs、胰蛋白酶、 caspases等的活性检测);
受体-配体结合分析:用AMC标记多肽配体,与细胞表面或纯化的受体孵育后,通过荧光成像可追踪配体与受体的结合过程,通过荧光强度定量结合亲和力(Kd值);
药物筛选:针对特定蛋白酶或受体的药物候选分子,以AMC标记多肽为探针,通过荧光信号的变化(如酶活性被抑制时荧光释放减少),高通量筛选药物的抑制/激活活性,评估药物 potency;
多肽体内/体外追踪:AMC标记的多肽进入细胞或生物体内后,可通过荧光显微镜、小动物活体成像系统等设备,实时观察多肽的分布、转运及代谢过程,为多肽药物的药代动力学研究提供支撑。
标记位点选择:必须避开多肽的生物活性中心(如酶切位点、受体结合位点),否则会导致多肽失去原有功能;若多肽含多个氨基/羧基,可通过控制反应摩尔比、pH值实现特异性单点标记;
反应条件控制:缓冲液pH需严格控制在7.0-8.0(过酸会抑制酰胺化反应,过碱会导致AMC活性酯水解失效);低温反应(4℃)可减少多肽降解,适合不稳定多肽的标记;
杂质去除:游离AMC会导致背景荧光偏高,影响检测准确性,因此标记后需通过HPLC彻底纯化,确保标记多肽纯度≥95%(科研级)或≥98%(药物研发级);
多肽稳定性保护:若多肽含半胱氨酸(Cys),需在反应体系中添加少量还原剂(如DTT),避免Cys侧链巯基氧化形成二硫键,影响多肽结构与标记效率;
荧光检测条件匹配:检测时需根据AMC的光学特性调整激发/发射波长,避免与实验体系中其他荧光物质(如细胞自身荧光、染料)的光谱重叠,减少干扰。
此前你关注过多肽AFC标记,两者核心差异在于荧光基团的结构与性能,具体对比如下:AMC含“甲基(-CH₃)”,AFC含“三氟甲基(-CF₃)”;AFC的背景荧光更低、光稳定性更强,但合成成本更高;AMC性价比更高,荧光信号强度足够满足多数科研需求,是更常用的基础荧光标记基团。
| DOI | 名称 | |
|---|---|---|
| 10.1038/sj.cdd.4401493 | Cathepsin B-independent abrogation of cell death by CA-074-OMe upstream of lysosomal breakdown | 下载 |
| 10.1016/j.jdermsci.2007.06.008 | Flavonolignans from Silybum marianum moderate UVA-induced oxidative damage to HaCaT keratinocytes | 下载 |
| 10.1182/blood-2009-10-247106 | Glucocorticoids promote survival of anti-inflammatory macrophages via stimulation of adenosine receptor A3 | 下载 |
| 10.1074/jbc.M108390200 | Dominant-negative suppression of HNF-1 alpha results in mitochondrial dysfunction, INS-1 cell apoptosis, and increased sensitivity to ceramide-, but not to high glucose-induced cell death | 下载 |
| 10.1046/j.1471-4159.2003.01724.x | Blockade of ionotropic glutamate receptors produces neuronal apoptosis through the Bax-cytochrome C-caspase pathway: the causative role of Ca2+ deficiency | 下载 |
| 10.1515/BC.2003.045 | Calpastatin exon 1B-derived peptide, a selective inhibitor of calpain: enhancing cell permeability by conjugation with penetratin | 下载 |
| 10.1167/iovs.02-1178 | Characterization of daunorubicin-induced apoptosis in retinal pigment epithelial cells: modulation by CD95L | 下载 |
| 10.1046/j.1471-4159.2003.02000.x | The amyloid precursor protein protects PC12 cells against endoplasmic reticulum stress-induced apoptosis | 下载 |
| 10.1007/s00705-003-0213-7 | AZT inhibits Visna/maedi virus-induced apoptosis | 下载 |
| 10.1016/S0014-5793(04)00276-5 | The inhibition of cell spreading on a cellulose substrate (cuprophan) induces an apoptotic process via a mitochondria-dependent pathway | 下载 |
| 10.1111/j.1478-3231.2004.0914.x | Hepatocyte survival depends on beta1-integrin-mediated attachment of hepatocytes to hepatic extracellular matrix | 下载 |
| 10.1186/1471-2407-4-54 | Opposite role of Bax and BCL-2 in the anti-tumoral responses of the immune system | 下载 |
| 10.1016/j.molcel.2004.10.028 | The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA | 下载 |
| 10.1038/sj.cdd.4401495 | Regulation of gene expression by the amyloid precursor protein: inhibition of the JNK/c-Jun pathway | 下载 |
| 10.1038/sj.leu.2403701 | Can application of serine protease inhibitors TPCK and TLCK provide evidence for possible involvement of serine protease Omi/HtrA2 in imatinib mesylate-induced cell death of BCR-ABL-positive human leukemia cells? | 下载 |
| 10.1016/j.febslet.2005.02.079 | Caspase-3 can be pseudo-activated by a Ca2+-dependent proteolysis at a non-canonical site | 下载 |
| 10.1074/jbc.M501092200 | Tyrosine phosphorylation regulates the proteolytic activation of protein kinase Cdelta in dopaminergic neuronal cells | 下载 |
| 10.1016/j.yjmcc.2005.09.012 | Trimetazidine inhibits mitochondrial permeability transition pore opening and prevents lethal ischemia-reperfusion injury | 下载 |
| 10.1038/sj.bjp.0706811 | Protective effects of relaxin in ischemia/reperfusion-induced intestinal injury due to splanchnic artery occlusion | 下载 |
| 10.1111/j.1349-7006.2006.00294.x | Role of phosphatidylinositol-3 kinase/Akt pathway in bladder cancer cell apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand | 下载 |
| 10.1038/sj.bjp.0707237 | Cell signaling pathways in the mechanisms of neuroprotection afforded by bergamot essential oil against NMDA-induced cell death in vitro | 下载 |
| 10.1111/j.1471-4159.2007.04750.x | Cocaine exposure in vitro induces apoptosis in fetal locus coeruleus neurons through TNF-alpha-mediated induction of Bax and phosphorylated c-Jun NH(2)-terminal kinase | 下载 |
| 10.4049/jimmunol.179.9.6024 | Geranylgeranylation but not GTP loading determines rho migratory function in T cells | 下载 |
| 10.1074/jbc.M704185200 | Metacaspase-8 modulates programmed cell death induced by ultraviolet light and H2O2 in Arabidopsis | 下载 |
| 10.1007/s00775-008-0344-0 | Inhibition of cathepsin B by Au(I) complexes: a kinetic and computational study | 下载 |
| 10.1002/0471140864.ps2112s27 | Assaying proteases in cellular environments | 下载 |
| 10.1186/1756-6606-1-12 | Environmental neurotoxin dieldrin induces apoptosis via caspase-3-dependent proteolytic activation of protein kinase C delta (PKCdelta): Implications for neurodegeneration in Parkinson's disease | 下载 |
| 10.1016/j.etp.2008.09.007 | Heart lesions associated with anabolic steroid abuse: comparison of post-mortem findings in athletes and norethandrolone-induced lesions in rabbits | 下载 |
| 10.1016/j.immuni.2008.10.017 | Fatal hepatitis mediated by tumor necrosis factor TNFalpha requires caspase-8 and involves the BH3-only proteins Bid and Bim | 下载 |
| 10.1074/jbc.M809671200 | Alpha-synuclein aggregation and Ser-129 phosphorylation-dependent cell death in oligodendroglial cells | 下载 |
| 10.1215/15228517-2009-012 | Cilengitide modulates attachment and viability of human glioma cells, but not sensitivity to irradiation or temozolomide in vitro | 下载 |
| 10.1038/sj.bjc.6605220 | Clinical significance of stromal apoptosis in colorectal cancer | 下载 |
| 10.1242/jcs.050963 | Toxoplasma gondii infection confers resistance against BimS-induced apoptosis by preventing the activation and mitochondrial targeting of pro-apoptotic Bax | 下载 |
| 10.1371/journal.pone.0009826 | Stress-induced sphingolipid signaling: role of type-2 neutral sphingomyelinase in murine cell apoptosis and proliferation | 下载 |
| 10.1007/978-1-60761-697-9_2 | Death and survival signals in photodynamic therapy | 下载 |
| 10.1371/journal.pone.0013638 | Caspase-10-dependent cell death in Fas/CD95 signalling is not abrogated by caspase inhibitor zVAD-fmk | 下载 |
| 10.1186/1471-2407-10-602 | Antiproliferative and pro-apoptotic effects afforded by novel Src-kinase inhibitors in human neuroblastoma cells | 下载 |
| 10.1186/1476-4598-9-298 | The novel sigma-2 receptor ligand SW43 stabilizes pancreas cancer progression in combination with gemcitabine | 下载 |
| 10.1016/j.chembiol.2010.08.014 | Identification and evaluation of small molecule pan-caspase inhibitors in Huntington's disease models | 下载 |
| 10.1016/j.vetimm.2010.11.001 | Immunological parameters in goats experimentally infected with SRLV genotype E, strain Roccaverano | 下载 |
| 10.1016/j.neurobiolaging.2011.06.012 | The APP intracellular domain (AICD) potentiates ER stress-induced apoptosis | 下载 |
| 10.1111/j.1582-4934.2012.01530.x | The non-anticoagulant heparin-like K5 polysaccharide derivative K5-N,OSepi attenuates myocardial ischaemia/reperfusion injury | 下载 |
| 10.1111/j.1600-0854.2012.01348.x | A centronuclear myopathy--dynamin 2 mutation impairs autophagy in mice | 下载 |
| 10.1186/1756-9966-31-41 | Lysosomal membrane permeabilization is an early event in Sigma-2 receptor ligand mediated cell death in pancreatic cancer | 下载 |
| 10.1111/j.1582-4934.2012.01595.x | Monocytic microparticles promote atherogenesis by modulating inflammatory cells in mice | 下载 |
| 10.4161/auto.20763 | Spatiotemporal autophagic degradation of oxidatively damaged organelles after photodynamic stress is amplified by mitochondrial reactive oxygen species | 下载 |
| 10.1155/2012/785786 | Combined Stimulation with the Tumor Necrosis Factor α and the Epidermal Growth Factor Promotes the Proliferation of Hepatocytes in Rat Liver Cultured Slices | 下载 |
| 10.1016/j.bcp.2013.01.022 | Autophagy takes place in mutated p53 neuroblastoma cells in response to hypoxia mimetic CoCl(2) | 下载 |
| 10.1515/hsz-2012-0213 | Cysteine cathepsins are not critical for TRAIL- and CD95-induced apoptosis in several human cancer cell lines | 下载 |
| 10.1038/bcj.2013.17 | Stimulation of Toll-like receptor-1/2 combined with Velcade increases cytotoxicity to human multiple myeloma cells | 下载 |
| 10.1007/s00441-014-1823-y | Effects of the chemokine CXCL12 and combined internalization of its receptors CXCR4 and CXCR7 in human MCF-7 breast cancer cells | 下载 |
| 10.1371/journal.pone.0154590 | Porphyromonas gingivalis Differentially Modulates Cell Death Profile in Ox-LDL and TNF-α Pre-Treated Endothelial Cells | 下载 |
| 10.1042/BJ20021017 | An intracellular protease of the crenarchaeon Sulfolobus solfataricus, which has sequence similarity to eukaryotic peptidases of the CD clan | 下载 |
| 10.1002/cyto.a.10059 | Sensitive and reliable JC-1 and TOTO-3 double staining to assess mitochondrial transmembrane potential and plasma membrane integrity: interest for cell death investigations | 下载 |
| 10.1016/j.bbrc.2004.02.021 | Caspase 3 activation is controlled by a sequence located in the N-terminus of its large subunit | 下载 |
| 10.1016/j.bcp.2004.10.010 | Impaired activation of caspases and prevention of mitochondrial dysfunction in the metastatic colon carcinoma CC531s-m2 cell line | 下载 |
| 10.1038/sj.leu.2403750 | STAT3 is essential for Hodgkin lymphoma cell proliferation and is a target of tyrphostin AG17 which confers sensitization for apoptosis | 下载 |
| 10.1111/j.1462-5822.2005.00533.x | Multiple virulence factors are required for Staphylococcus aureus-induced apoptosis in endothelial cells | 下载 |
| 10.1002/jcb.21550 | Serine protease inhibitors N-alpha-tosyl-L-lysinyl-chloromethylketone (TLCK) and N-tosyl-L-phenylalaninyl-chloromethylketone (TPCK) are potent inhibitors of activated caspase proteases | 下载 |
| 10.1016/j.bbamcr.2009.03.007 | Palmitoyl protein thioesterase 1 modulates tumor necrosis factor alpha-induced apoptosis | 下载 |
| 10.18632/oncotarget.4067 | p53 attenuates AKT signaling by modulating membrane phospholipid composition | 下载 |
多肽Ac-Asp-Glu-Val-Asp-AMC的合成步骤:
1、合成CTC树脂:称取2.97g CTC Resin(如初始取代度约为0.41mmol/g)和1.46mmol Fmoc-Asp(OtBu)-OH于反应器中,加入适量DCM溶解氨基酸(需要注意,此时CTC树脂体积会增大好几倍,避免DCM溶液过少),再加入3.65mmol DIPEA(Mw:129.1,d:0.740g/ml),反应2-3小时后,可不抽滤溶液,直接加入1ml的HPLC级甲醇,封端半小时。依次用DMF洗涤2次,甲醇洗涤1次,DCM洗涤一次,甲醇洗涤一次,DCM洗涤一次,DMF洗涤2次(这里使用甲醇和DCM交替洗涤,是为了更好地去除其他溶质,有利于后续反应)。得到 Fmoc-Asp(OtBu)-CTC Resin。结构图如下:
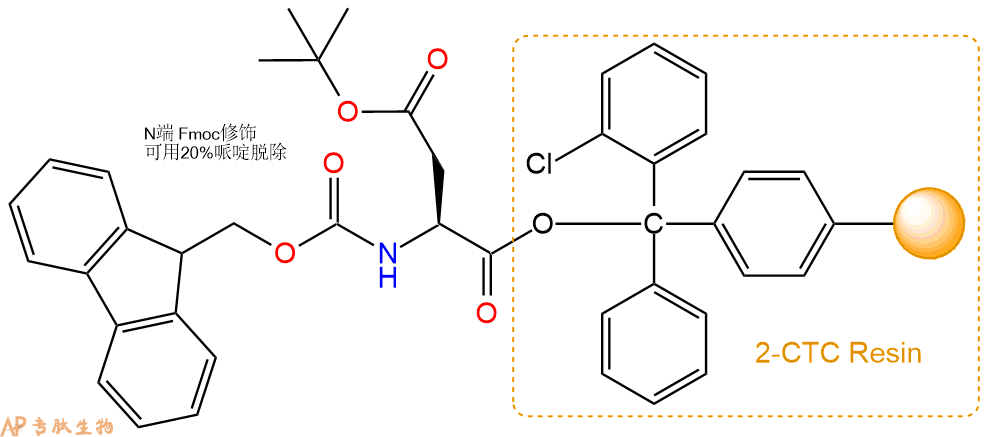
2、脱Fmoc:加3倍树脂体积的20%Pip/DMF溶液,鼓氮气30分钟,然后2倍树脂体积的DMF 洗涤5次。得到 H2N-Asp(OtBu)-CTC Resin 。(此步骤脱除Fmoc基团,茚三酮检测为蓝色,Pip为哌啶)。结构图如下:
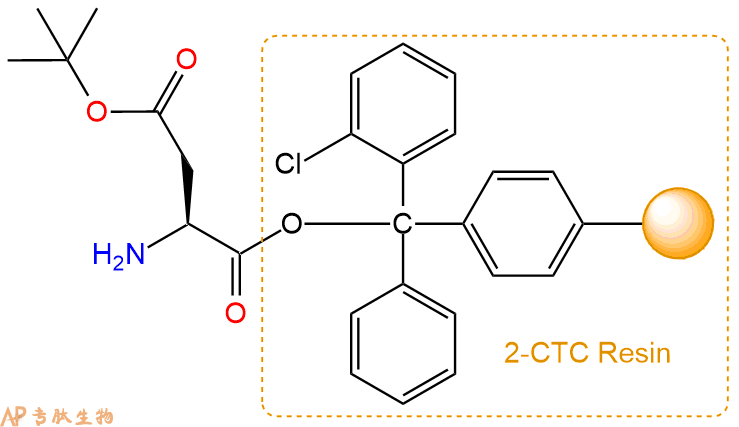
3、缩合:取3.65mmol Fmoc-Val-OH 氨基酸,加入到上述树脂里,加适当DMF溶解氨基酸,再依次加入7.31mmol DIPEA,3.47mmol HBTU。反应30分钟后,取小样洗涤,茚三酮检测为无色。用2倍树脂体积的DMF 洗涤3次树脂。(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。得到Fmoc-Val-Asp(OtBu)-CTC Resin。氨基酸:DIPEA:HBTU:树脂=3:6:2.85:1(摩尔比)。结构图如下:
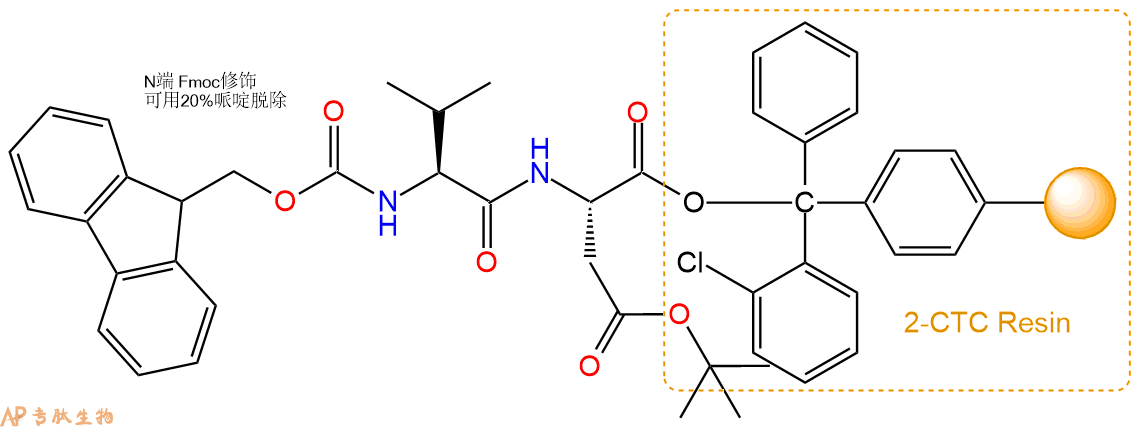
4、依次循环步骤二、步骤三,依次得到
H2N-Val-Asp(OtBu)-CTC Resin
Fmoc-Glu(OtBu)-Val-Asp(OtBu)-CTC Resin
H2N-Glu(OtBu)-Val-Asp(OtBu)-CTC Resin
Fmoc-Asp(OtBu)-Glu(OtBu)-Val-Asp(OtBu)-CTC Resin
以上中间结构,均可在专肽生物多肽计算器-多肽结构计算器中,一键画出。
最后再经过步骤二得到 H2N-Asp(OtBu)-Glu(OtBu)-Val-Asp(OtBu)-CTC Resin,结构如下:
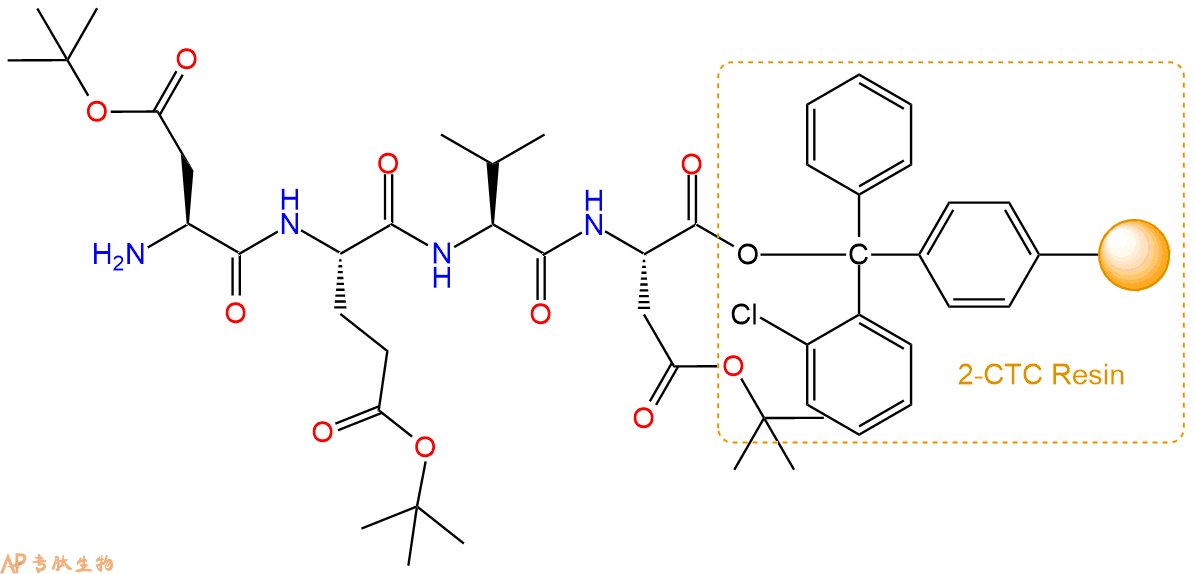
5、乙酸酐反应连接:在上述树脂中,加入适当DMF后,再加入3.65mmol 乙酸酐到树脂中,再加入7.31mmol DIPEA、3.47mmol HBTU,鼓氮气反应30分钟。用2倍树脂体积的DMF 洗涤3次树脂(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。 得到Ac-Asp(OtBu)-Glu(OtBu)-Val-Asp(OtBu)-CTC Resin。 结构如下:
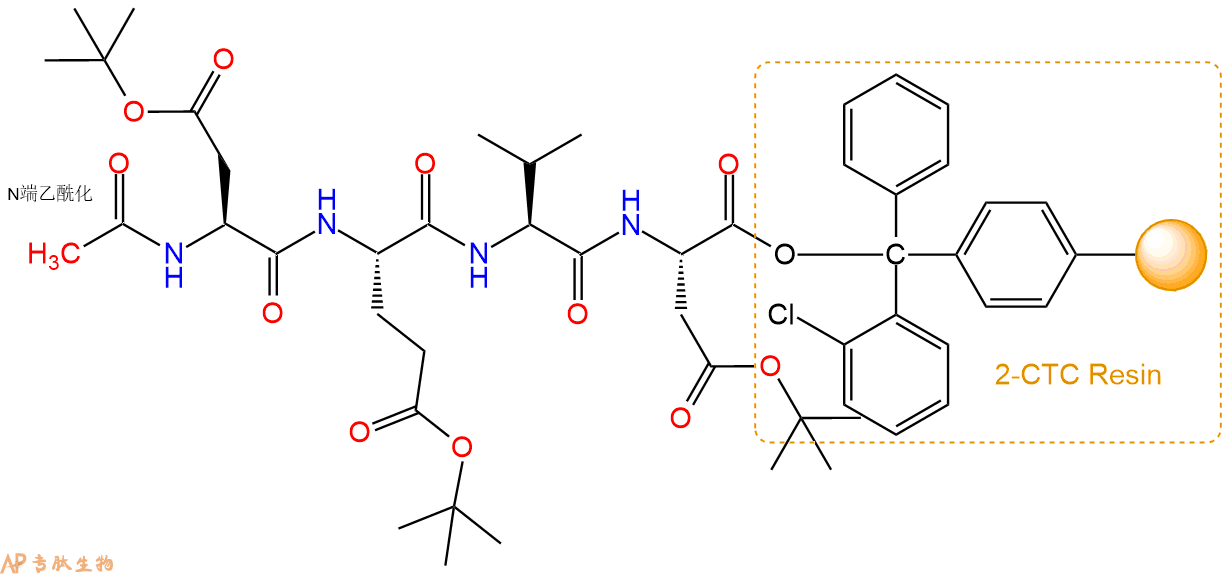
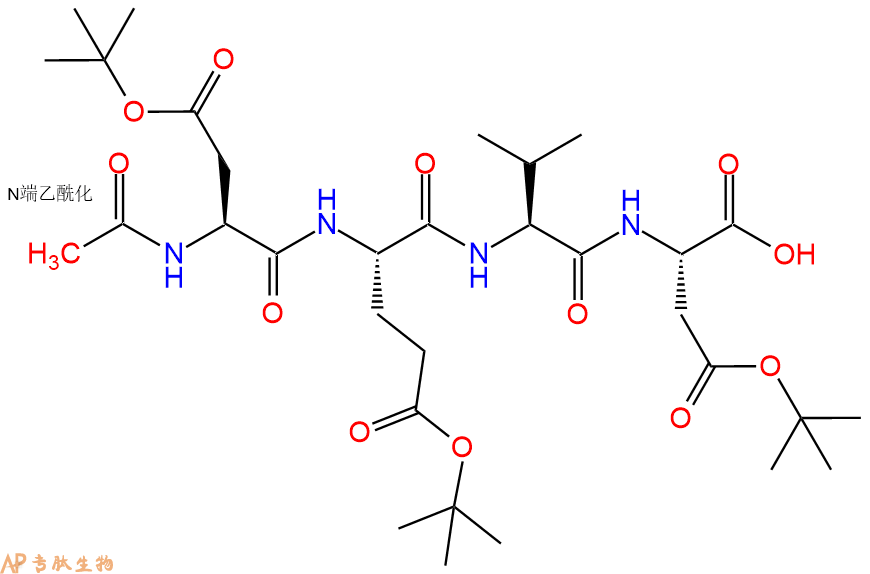
6、全保护切割:配置0.5%TFA/DCM溶液,溶液体积约为树脂体积的3倍。再次用DCM洗涤树脂2遍(去除残留DMF),后将配置好的溶液倒入到反应器中,反应30分钟。抽滤树脂,收集滤液(此时多肽已经从树脂上分离,存在于滤液中)。多肽序列为 Ac-Asp(OtBu)-Glu(OtBu)-Val-Asp(OtBu)-CTC Resin。 在滤液中添加DIEPA,调PH至7-8。用饱和NaHCO3洗涤滤液,分离出DCM层溶液。可适当旋蒸DCM层溶液,减少有机溶剂。再次加入1或2倍体积的乙酸乙酯,用稀HCl溶液调PH至微酸性,将多肽从DCM层萃取到乙酸乙酯层。用饱和NaCl洗涤2次乙酸乙酯层。用无水硫酸镁吸收乙酸乙酯层的水分。通过减压旋蒸,直接将乙酸乙酯完全旋蒸掉,得到晶体状固体多肽,用于下一步C端反应。或通过减压旋蒸保留适量乙酸乙酯的溶液体积,加入冰乙醚析出 多肽,然后对多肽进行烘干操作即可用于下一步C端反应。Ac-Asp(OtBu)-Glu(OtBu)-Val-Asp(OtBu)-COOH的结构图如下。
7、7-氨基-4-甲基香豆素反应连接:在上述树脂中,加入适当DMF后,再加入3.65mmol 7-氨基-4-甲基香豆素到树脂中,再加入7.31mmol DIPEA、3.47mmol HBTU,鼓氮气反应30分钟。用2倍树脂体积的DMF 洗涤3次树脂(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。 得到 Ac-Asp(OtBu)-Glu(OtBu)-Val-Asp(OtBu)-AMC。 结构如下:
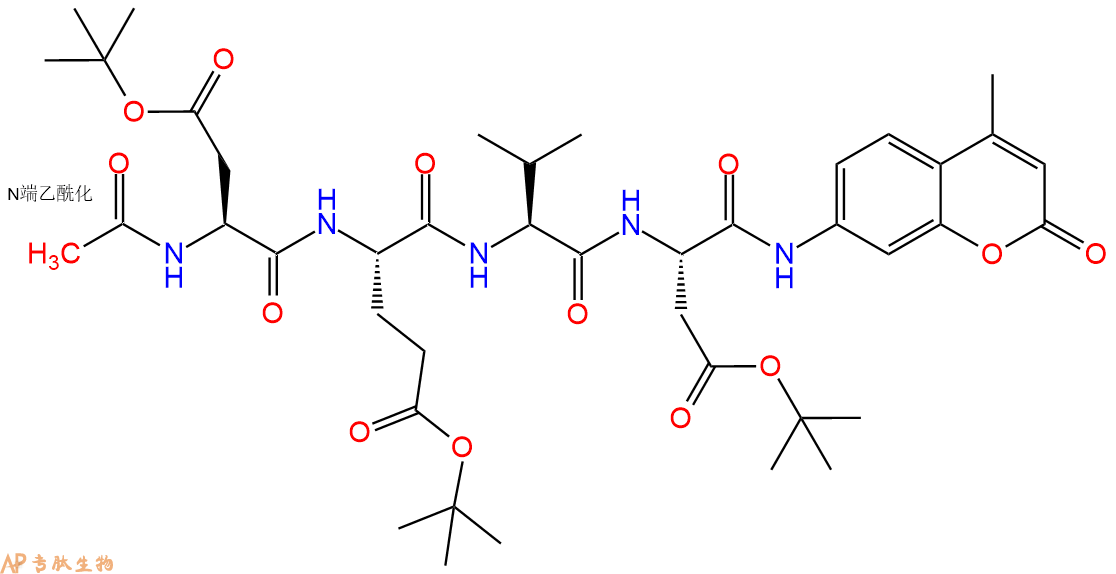
8、切割:6倍树脂体积的切割液(或每1g树脂加8ml左右的切割液),摇床摇晃 2小时,过滤掉树脂,用冰无水乙醚沉淀滤液,并用冰无水乙醚洗涤沉淀物3次,最后将沉淀物放真空干燥釜中,常温干燥24小试,得到粗品Ac-Asp-Glu-Val-Asp-AMC。结构图见产品结构图。
切割液选择:1)TFA:H2O=95%:5%、TFA:H2O=97.5%:2.5%
2)TFA:H2O:TIS=95%:2.5%:2.5%
3)三氟乙酸:茴香硫醚:1,2-乙二硫醇:苯酚:水=87.5%:5%:2.5%:2.5%:2.5%
(前两种适合没有容易氧化的氨基酸,例如Trp、Cys、Met。第三种适合几乎所有的序列。)
9、纯化冻干:使用液相色谱纯化,收集目标峰液体,进行冻干,获得蓬松的粉末状固体多肽。不过这时要取小样复测下纯度 是否目标纯度。
10、最后总结:
杭州专肽生物技术有限公司(ALLPEPTIDE https://www.allpeptide.com)主营定制多肽合成业务,提供各类长肽,短肽,环肽,提供各类修饰肽,如:荧光标记修饰(CY3、CY5、CY5.5、CY7、FAM、FITC、Rhodamine B、TAMRA等),功能基团修饰肽(叠氮、炔基、DBCO、DOTA、NOTA等),同位素标记肽(N15、C13),订书肽(Stapled Peptide),脂肪酸修饰肽(Pal、Myr、Ste),磷酸化修饰肽(P-Ser、P-Thr、P-Tyr),环肽(酰胺键环肽、一对或者多对二硫键环),生物素标记肽,PEG修饰肽,甲基化修饰肽
以上所有内容,为专肽生物原创内容,请勿发布到其他网站上。





