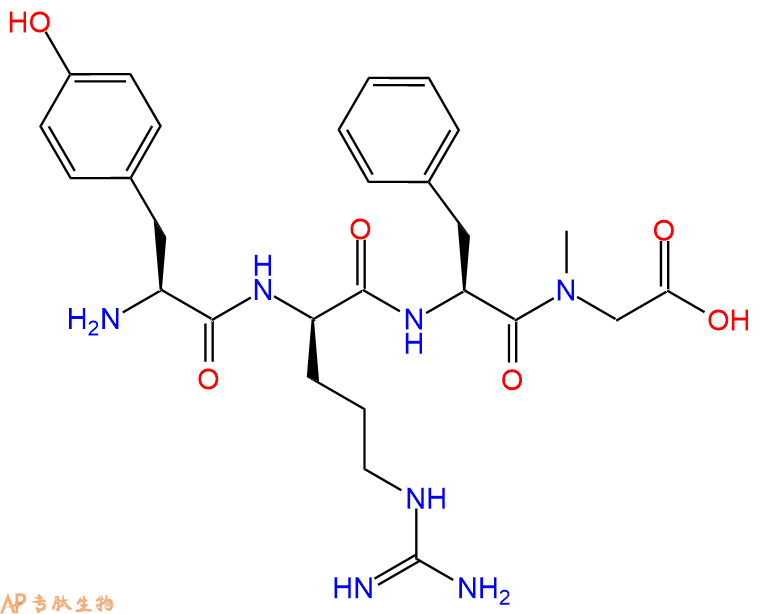
A proposed µ-opioid receptor selective tetrapeptide analog of dermorphin. It showed more potent and longer-lasting analgesic activities than morphine.
编号:143999
CAS号:90549-86-3
单字母:H2N-YrF-(NMe)G-OH
| 编号: | 143999 |
| 中文名称: | [DArg2, Sar4]Dermorphin(1-4) |
| 英文名: | [DArg2, Sar4]Dermorphin(1-4) |
| CAS号: | 90549-86-3 |
| 单字母: | H2N-YrF-(NMe)G-OH |
| 三字母: | H2N N端氨基 -Tyr酪氨酸 -DArgD型精氨酸 -Phe苯丙氨酸 -SarN甲基化甘氨酸 -OHC端羧基 |
| 氨基酸个数: | 4 |
| 分子式: | C27H37N7O6 |
| 平均分子量: | 555.63 |
| 精确分子量: | 555.28 |
| 等电点(PI): | - |
| pH=7.0时的净电荷数: | 2.97 |
| 平均亲水性: | -0.96666666666667 |
| 疏水性值: | -0.87 |
| 外观与性状: | 白色粉末状固体 |
| 消光系数: | 1490 |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 纯度: | 95%、98% |
| 盐体系: | 可选TFA、HAc、HCl或其它 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | D型氨基酸肽 甲基化修饰肽 皮啡肽(Dermorphin) |
A proposed µ-opioid receptor selective tetrapeptide analog of dermorphin. It showed more potent and longer-lasting analgesic activities than morphine.
很多蛋白在细胞中非常容易被降解,或被标记,进而被选择性地破坏。但含有部分D型氨基酸的多肽则显示了很强的抵抗蛋白酶降解能力。
甲基化修饰多肽
也叫甲基化标记多肽,甲基化修饰是蛋白质翻译后修饰(PTMs)的一种,几乎参与细胞所有的生命活动过程,发挥着重要的调控作用,蛋白质在甲基转移酶的催化下将甲基转移至特定的氨基酸残基上共价结合的过程。甲基化是一种可逆的修饰过程,由去甲基化酶催化去甲基化作用。
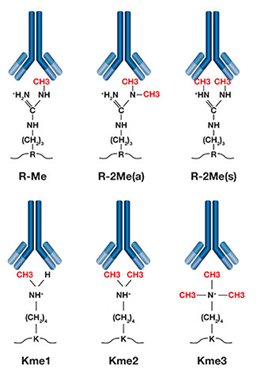
研究发现,常见甲基化/去甲基化作用的氨基酸主要是赖氨酸(Lys)和精氨酸(Arg)研究表明,组蛋白赖氨酸甲基化修饰执行着多种生物学功能,如干细胞的维持和分化、X染色体失活、转录调节和DNA损伤反应等,主要是影响染色质浓缩,抑制基因表达。组蛋白精氨酸甲基化在基因转录调控中发挥着重要作用,并能影响细胞的多种生理过程,包括DNA修复、信号转导、细胞发育及癌症发生等因此专肽生物特地开发甲基化修饰多肽技术,为科学家在蛋白质翻译后修饰(PTMS)的研究中提供帮助。
甲基化修饰(Me1,Me2,Me3)
采用高品质的Fmoc-Lys(Me,Boc)-OH、 Fmoc-Lys(Me2)-OH、Fmoc-Lys(Me3)-OH.HCL、Fmoc-Arg(Me,Pbf)-OH 、Fmoc-Arg(me)2-OH.HCl(asymmetrical) 、Fmoc-Arg(me)2-OH.HCl(symmetrical) 等原料,采用Fmoc固相合成工艺合成,得到Lys甲基化,Arg甲基化标记的多肽,使用HPLC 对产物进行纯化。最终产品提供相应的质谱图,纯度分析的HPLC 色谱图。
Definition
A group of opioid peptides has been discovered in the skin of South American frogs belonging to the subfamily Phyllomedusinae. The first peptide isolated from several species of these frogs was dermorphin which was shown to have high affinity and selectivity for µ-type opioid receptors 1.
Related Peptides
After the discovery of dermorphins, two additional peptides with even higher affinity for the d receptor were subsequently isolated from the skin of Phyllomedusa bicolor. Like dermorphin, these peptides contain D-alanine as the second amino acid and they have been termed [D-Ala2] deltorphins I and II 1. Dermorphins and deltorphins are heptapeptides with the common amino terminal sequence Tyr-D-Ala-Phe 2.
Discovery
In 1981, Montecucchi et al., extracted from the skin of the Argentinian frog Phyllomedusa sauvagei a heptapeptide named dermorphin, which preferentially binds to µ-type opioid receptors. Erspamer et al., in 1989 reported the isolation of deltorphins from the skin of P. sauvagei 2.
Structural Characteristics
These are heptapeptides with the common aminoterminal sequence Tyr-D-Ala-Phe. Since this initial sequence is conserved, it is presumed that it is necessary for binding to both µ and d sites of opioid receptors. Unlike dermorphin, the deltorphins have a charged amino acid in position 4, namely histidine in the case of deltorphin and aspartic or glutamic acid in the case of [D-Ala2]-deltorphins. The presence of positively or negatively charged amino acids in this position has little influence on the binding characteristics. It thus seems likely that other features in the C-terminal regions of deltorphins are essential for the observed receptor selectivity. Deltorphins are flexible linear peptides. They show an affinity for d sites 10 to 200 times higher than that of the synthetic enkephalin analogue 2. Deltorphins contain the amino terminal sequence Tyr-Ala-Phe, which is preceded by the typical prohormone processing signal Lys-Arg. Following the deltorphin sequence is the more complex processing sequence Gly-Glu-Ala-Lys-Lys, the glycine being required for the formation of the carboxyl-terminal amide. The same flanking sequences have also been found in the dermorphin precursors from Ph. sauvagei 1.
Analogs: D-Ala2, Glu4] - and [D-Ala2, Asp4] deltorphinamides, their L isomers, and 1-(3, 5-diiodotyrosyl) [D-Ala2] deltorphins are synthetic analogs of deltorphins prepared by solid-phase synthesis 2.
Dermorphin analogs, [Lys7-NH2] dermorphin, [Arg7-NH2] dermorphin, and [Asn7-NH2] dermorphin behave as potent analgesic agents. The antinociception effect of [Lys7-NH2] dermorphin lasts longer than that of dermorphin itself 3.
In another study, the influence of dermorphin analogs with stereochemical modification of the amino acid residue proline in position 6 (Pro6), Tyr-D-Ala-Phe-Gly-Tyr-Hyp-Ser-NH2, Tyr-D-Ala-Phe-Gly-Tyr-[D-Pro]-Ser-NH2, Tyr-D-Ala-Phe-Gly-Tyr-[dehydro-Pro]-Ser-NH2, and Tyr-D-Ala-Phe-Gly-Tyr-[D-dehydro-Pro]-Ser-NH2, was analysed after their intraperitoneal injection at 0.5 mg/kg dose in the cold (4–7°C), thermoneutral (27–28°C), and hot (31–33°C) environment. Stereochemical modifications of amino acid residue Pro6 proved to induce specific changes in the thermoregulatory effect of the peptide. Substitution of DPro6 for Pro6 has the most dramatic consequences: it considerably attenuated the thermoregulatory effect of dermorphin in the cold environment, cancelled it in the hot environment, and inverted the dermorphin-specific thermoregulatory response in thermoneutral conditions. The data thus obtained indicate the important role of Pro6 residue in realization of this physiological activity of dermorphins 4.
Mode of Action
Dermorphin and deltorphins have high affinity and selectivity for µ- and d-type opioid receptors, respectively 2.
Functions
Dermorphin peptides are potent analgesics in rodents and primates, including man. Some dermorphins can enter the blood-brain barrier and produce central antinociception after peripheral administration. The dermorphin family also includes µ 1-opioid receptor selective agonists that produce intense opioid analgesia, but stimulate pulmonary ventilation. Experiments in rats and mice chronically exposed to dermorphins have shown that not only do they have higher antinociceptive efficacy and potency than morphine, but they are also less likely than morphine to produce tolerance, dependence and opiate side effects 5. [3H][D-Ala2] deltorphin I is a valuable probe for binding studies, since its affinity and selectivity are the highest of all the d -selective ligands known to date 2.
References
1. K Richter K, R Egger R, L Negri L, R Corsi R, C Severini C, Kreil G (1990). cDNAs encoding [D-Ala2]deltorphin precursors from skin of Phyllomedusa bicolor also contain genetic information for three dermorphin-related opioid peptides (amphibian skin peptides / precursors). PNAS., 87(12)4836-4839.
2. Erspamer V, Melchiorri P, Falconieri-Erspamer G, Negri L, Corsi R, Severini C, Barra D, Simmaco M Kreil G (1989). Deltorphins: A family of naturally occurring peptides with high affinity and selectivity for d opioid binding sites (amphibian skin peptides/mouse vas deferens assay/receptor binding assay). PNAS., 86:188-5192.
3. Negri L, Erspamer GF, Severini C, Potenza RL, Melchiorri P, Erspamer V (1992). Dermorphin-related peptides from the skin of Phyllomedusa bicolor and their amidated analogs activate two µ opioid receptor subtypes that modulate antinociception and catalepsy in the rat. PNAS., 89:7203-7207.
4. Emel’yanova TG, Usenko AB, Bonartsev AP, Kamenskii AA, Guzevatykh LS, Andreeva LA, Alfeeva L, Myasoedov NF (2002). Effect of Dermorphin Analogs on Thermoregulation of Rats under Various Thermal Conditions. Biology Bulletin, 29(3):284–289.
5. Melchiorri P, Negri L. (1996). The dermorphin peptide family. Gen Pharmacol., 27(7):1099-1107.
| DOI | 名称 | |
|---|---|---|
| 10.1016/0006-291x(84)91435-9 | D-Arg2-dermorphin tetrapeptide analogs: a potent and long-lasting analgesic activity after subcutaneous administration | 下载 |
| 10.1016/s0014-2999(98)00296-9 | Electrophysiological and behavioral effects of Tyr-D-Arg-Phe-Sar on locus coeruleus neurons of the rat | 下载 |
多肽H2N-Tyr-DArg-Phe-Sar-COOH的合成步骤:
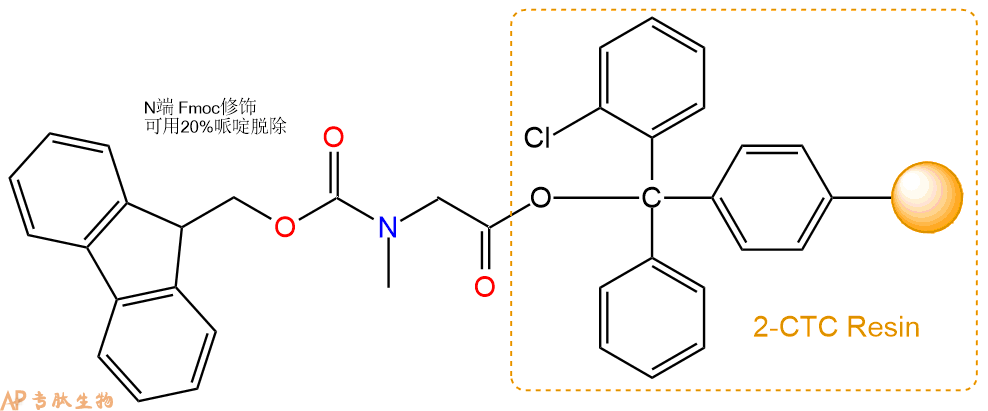
1、合成CTC树脂:称取0.7g CTC Resin(如初始取代度约为0.9mmol/g)和0.76mmol Fmoc-Sar-OH于反应器中,加入适量DCM溶解氨基酸(需要注意,此时CTC树脂体积会增大好几倍,避免DCM溶液过少),再加入1.89mmol DIPEA(Mw:129.1,d:0.740g/ml),反应2-3小时后,可不抽滤溶液,直接加入1ml的HPLC级甲醇,封端半小时。依次用DMF洗涤2次,甲醇洗涤1次,DCM洗涤一次,甲醇洗涤一次,DCM洗涤一次,DMF洗涤2次(这里使用甲醇和DCM交替洗涤,是为了更好地去除其他溶质,有利于后续反应)。得到 Fmoc-Sar-CTC Resin。结构图如下:
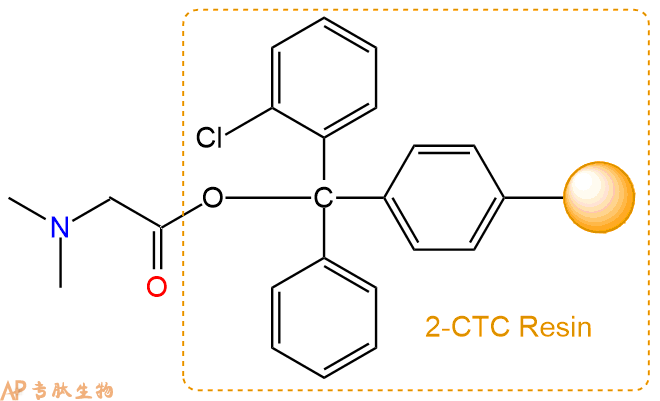
2、脱Fmoc:加3倍树脂体积的20%Pip/DMF溶液,鼓氮气30分钟,然后2倍树脂体积的DMF 洗涤5次。得到 H2N-Sar-CTC Resin 。(此步骤脱除Fmoc基团,茚三酮检测为蓝色,Pip为哌啶)。结构图如下:
3、缩合:取1.89mmol Fmoc-Phe-OH 氨基酸,加入到上述树脂里,加适当DMF溶解氨基酸,再依次加入3.78mmol DIPEA,1.8mmol HBTU。反应30分钟后,取小样洗涤,茚三酮检测为无色。用2倍树脂体积的DMF 洗涤3次树脂。(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。得到Fmoc-Phe-Sar-CTC Resin。氨基酸:DIPEA:HBTU:树脂=3:6:2.85:1(摩尔比)。结构图如下:
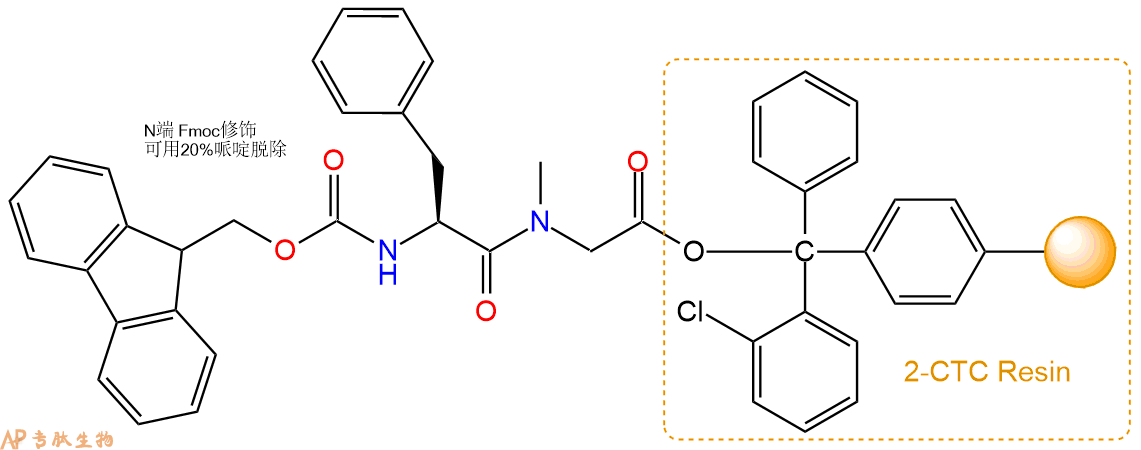
4、依次循环步骤二、步骤三,依次得到
H2N-Phe-Sar-CTC Resin
Fmoc-DArg(Pbf)-Phe-Sar-CTC Resin
H2N-DArg(Pbf)-Phe-Sar-CTC Resin
Fmoc-Tyr(tBu)-DArg(Pbf)-Phe-Sar-CTC Resin
以上中间结构,均可在专肽生物多肽计算器-多肽结构计算器中,一键画出。
最后再经过步骤二得到 H2N-Tyr(tBu)-DArg(Pbf)-Phe-Sar-CTC Resin,结构如下:

5、切割:6倍树脂体积的切割液(或每1g树脂加8ml左右的切割液),摇床摇晃 2小时,过滤掉树脂,用冰无水乙醚沉淀滤液,并用冰无水乙醚洗涤沉淀物3次,最后将沉淀物放真空干燥釜中,常温干燥24小试,得到粗品H2N-Tyr-DArg-Phe-Sar-COOH。结构图见产品结构图。
切割液选择:1)TFA:H2O=95%:5%、TFA:H2O=97.5%:2.5%
2)TFA:H2O:TIS=95%:2.5%:2.5%
3)三氟乙酸:茴香硫醚:1,2-乙二硫醇:苯酚:水=87.5%:5%:2.5%:2.5%:2.5%
(前两种适合没有容易氧化的氨基酸,例如Trp、Cys、Met。第三种适合几乎所有的序列。)
6、纯化冻干:使用液相色谱纯化,收集目标峰液体,进行冻干,获得蓬松的粉末状固体多肽。不过这时要取小样复测下纯度 是否目标纯度。
7、最后总结:
杭州专肽生物技术有限公司(ALLPEPTIDE https://www.allpeptide.com)主营定制多肽合成业务,提供各类长肽,短肽,环肽,提供各类修饰肽,如:荧光标记修饰(CY3、CY5、CY5.5、CY7、FAM、FITC、Rhodamine B、TAMRA等),功能基团修饰肽(叠氮、炔基、DBCO、DOTA、NOTA等),同位素标记肽(N15、C13),订书肽(Stapled Peptide),脂肪酸修饰肽(Pal、Myr、Ste),磷酸化修饰肽(P-Ser、P-Thr、P-Tyr),环肽(酰胺键环肽、一对或者多对二硫键环),生物素标记肽,PEG修饰肽,甲基化修饰肽
以上所有内容,为专肽生物原创内容,请勿发布到其他网站上。





