一种致脑肽,可诱导碱性蛋白特异性 T 细胞增殖。在外周血单核细胞中引起 Th1 极化,与多发性硬化症 (MS) 有关。
编号:172728
CAS号:118506-26-6
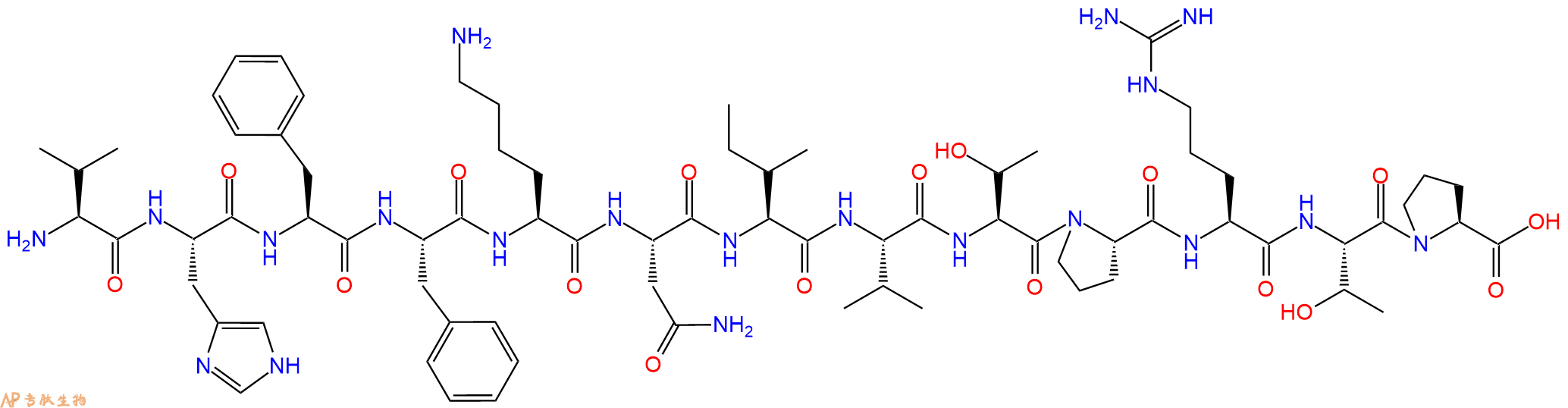
单字母:H2N-VHFFKNIVTPRTP-OH
| 编号: | 172728 |
| 中文名称: | Myelin Basic Protein (87-99), human |
| 英文名: | MyelinBasic Protein (87-99), human |
| 英文同义词: | MBP (87 - 99), human |
| CAS号: | 118506-26-6 |
| 单字母: | H2N-VHFFKNIVTPRTP-OH |
| 三字母: | H2N N端氨基 -Val缬氨酸 -His组氨酸 -Phe苯丙氨酸 -Phe苯丙氨酸 -Lys赖氨酸 -Asn天冬酰胺 -Ile异亮氨酸 -Val缬氨酸 -Thr苏氨酸 -Pro脯氨酸 -Arg精氨酸 -Thr苏氨酸 -Pro脯氨酸 -OHC端羧基 |
| 氨基酸个数: | 13 |
| 分子式: | C74H114N20O17 |
| 平均分子量: | 1555.82 |
| 精确分子量: | 1554.87 |
| 等电点(PI): | - |
| pH=7.0时的净电荷数: | 3.21 |
| 酸性基团个数: | 2.1 |
| 碱性基团个数: | 疏水 |
| 平均亲水性: | -0.44545454545455 |
| 疏水性值: | -0.09 |
| 外观与性状: | 白色粉末状固体 |
| 闪点: | 0 M-1cm-1 |
| 消光系数: | - |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 纯度: | 95%、98% |
| 盐体系: | 可选TFA、HAc、HCl或其它 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 脑脊髓炎(Experimental Allergic Encephalomyelitis (EAE) |
Myelin Basic Protein(87-99) TFA 是一种致脑肽,可诱导碱性蛋白特异性 T 细胞增殖。Myelin Basic Protein(87-99) TFA 在外周血单核细胞中引起 Th1 极化,与多发性硬化症 (MS) 有关。
Myelin Basic Protein(87-99) TFA is an encephalitogenic peptide that induces basic protein-specific T cell proliferation. Myelin Basic Protein(87-99) TFA causes a Th1 polarization in peripheral blood mononuclear cells with is implicated of multiple sclerosis (MS).
髓磷脂碱性蛋白(87-99)醋酸盐是一种致脑肽,在中枢神经系统中诱导T细胞增殖和Th1极化。髓磷脂碱性蛋白(MBP)是一种潜在的靶抗原,因为它在易感动物中诱导实验性变态反应性脑脊髓炎(EAE)。
Myelin Basic Protein (87-99) acetate is an encephalitogenic peptide that induces T cell proliferation with Th1 polarization in the CNS. Myelin basic protein (MBP) is a potential target antigen because it induces experimental allergic encephalomyelitis (EAE) in susceptible animals.
Experimental Allergic Encephalomyelitis (EAE) Peptides are active fragment of the myelin basic protein. By a cellmediated immune response, the peptide causes experimental allergic encephalomyelitis, which is an inflammatory demyelinating disease of the central nervous system. These peptides have been used as a model for studying multiple sclerosis (MS) due to the clinical and histopathological similarities of the inflammatory diseases affecting the central nervous system. Both Myelin PLP (PLP-3602-PI) and MOG (PMG-3660-PI) are antigenic peptides that induce EAE by binding to MHCII molecules on antigen presenting cells where they are recognized by class-II restricted T cells.
Discovery
Westall et al., in 1971 identified a peptide that causes experimental allergic encephalomyelitis 1,2. EAE is an autoimmune disease inducible by encephalitogenic helper T cells expressing Vβ8. Owhashi M et al., in 1997 examined the relationship between the stressor-induced alternation of clinical EAE and the induction of autoreactive T cells using Lewis rats 3.
Structural Characteristics
Belogurov AA et al demonstrated that autoantibodies (AAb) in multiple sclerosis (MS) reveal site-specific binding and cleavage toward myelin basic protein (MBP) epitope library. They have found several fragments of MBP immunodominant in terms of AAb binding and applied these peptides to DA rats with induced protracted relapsing EAE most closely related to MS. DA rats with EAE induced by syngenic spinal cord homogenate in complete Freund's adjuvant were treated by nasal route with human MBP 46–62, 81–102, 124–139, 147–170, and Copaxone®. MBP 124–139 and 147–170 displayed only mild therapeutic effects but MBP 46–62 significantly reduced EAE, reflected by lower clinical scores and shorter EAE duration compared to controls 4. Three peptides overlapping the tryptophan region of bovine CNS myelin basic protein were synthesized by the solid phase procedure of Merrifield. These were the nonapeptide H-Phe-Ser-Trp-Gly-Ala-Glu-Gly-Gln-Lys-OH, the octapeptide H-Ser-Trp-Gly-Ala-Glu-Gly-Gln-Lys-OH, and the heptapeptide H-Trp-Gly-Ala-Glu-Gly-Gln-Lys-OH. They were tested fro encephalitogenic activity in guinea pigs with either Freund's complete adjuvant containing M. tuberculosis or muramyl dipeptide in incomplete Freud's adjuvant at doses of 10 µg per animal. The results show that deletion of one or two residues from the amino-terminal end of the nonapeptide destroyed the ability of the shorter peptides to induce clinical but not histological signs of EAE 5.
Mode of Action
Proteolipid protein (PLP) is the major protein of central nervous system (CNS) myelin. SJL(H-2S) mice immunized with a synthetic peptide corresponding to PLP residues 139-151 (HSLGKWLGHPDKF) develop acute EAE. A T cell line and 4 clones were derived from SJL/J mice were immunizied with this synthetic peptide. Severe clinical and histological EAE was induced by adoptive transfer of the peptide-specific T cell line and 3 of 4 T cell clones. The T cell line/clones all responded strongly to PLP peptide 139-151 in in vitro proliferative assays. However, two different reactivity patterns emerged when truncated PLP peptides 141-150 and 141-149 were tested, suggesting that more than 1 epitope may be present within the PLP 139-151 determinant. Two truncated PLP peptides were compared for the ability to induce EAE in vivo and proliferative responses in vitro. Immunization with PLP peptide 141-150 induced acute EAE in about 70% of mice tested, but PLP peptide 141-149 induced a comparatively mild form of EAE in 4 out of 9 mice tested. Lymph node cells from mice immunized with these peptides showed in vitro proliferative responses to each of the peptides, but the response to peptide 139-151 was always strongest. These combined in vivo and in vitro data further define the epitopes involved in PLP-induced EAE in SJL mice. Furthermore, the availability of multiple PLP-specific T cell clones will enable to study the diversity of the T cell repertoire to PLP 6.
Functions
Cell mediated autoimmune response, EAE is a cell mediated autoimmune response directed against autologous central nervous system myelin 7.
Sensitization with myelin basic protein (MBP), a major protein constituent of central nervous system compact myelin, emulsified in complete Freund's adjuvant, produce the full clinical and histological picture of EAE in a wide variety of animal.
The encephalitogenic determinants responsible for EAE induction are species-specific. That is, different sequences of amino acid residues located at unique positions within the MBP molecule are critical for the induction of EAE in each mammalian species 8.
Stressor-induced suppression of clinical EAE is not simply because of the failure of induction of autoreactive T cells, nor localization of the autoreactive T cells in the central nervous system 3.
Species-specific immune response, the capacity of MBP in complete Freund's adjuvant to induce an encephalitogenic immune response against autologous central nervous system myelin in a given mammalian species appears to be dictated by latent, species-specific immune response genes which presumably encode antigen-receptor molecules recognizing specific MBP sequences 1.
References
Westall FC, Robinson AB, Caccam J, Jackson J, Ylar EH, (1971). Essential chemical requirements for induction of allergic encephalomyelitis. Nature, 229(5279):22-24.
Shapira R, Chou FC, McKneally S, Urban E, Kibler RF (1971). Biologicl activity and synthesis of an encephalitogenic determinant. Science, 173(998):736-738.
Owhashi M, Shouzui Y, Arita H (1997). Stress down-regulates experimental allergic encephalomyelitis (EAE) but permits activation and localization of autoreactive vβ8.2+ T Cells. International Journal of Neuroscience, 89(3-4):177-188.
Belogurov AA Jr, Zargarova TA, Turobov VI, Novikova NI, Favorova OO, Ponomarenko NA, Gabibov AG (2009). Suppression of ongoing experimental allergic encephalomyelitis in DA rats by novel peptide drug, structural part of human myelin basic protein 46–62. Autoimmunity, 42(4):362-364.
Levit S, Powers JM, Milek D, Brostoff SW (1980). Peptide length requirement for experimental allergic encephalomyelitis in guinea pigs. Neurochem Res., 5(1):37-42.
Kuchroo VK, Sobel RA, Yamamura T, Greenfield E, Dorf ME, Lees MB (1991). Induction of Experimental Allergic Encephalomyelitis by Myelin Proteolipid-Protein-Specific T Cell Clones and Synthetic Peptides. Pathobiology, 59(5):305-312.
Paterson PY (1982). Molecular and cellular determinants of neuroimmunologic inflammatory disease. Fed. Proc. Fed. Am. Soc. Exp. Biol., 41:2569-2576.
Hashim GA (1978). Myelin basic protein: structure, function and antigenic determinants. Immunol. Rev., 39:60-107.
R E Jones, et al. The synthetic 87-99 peptide of myelin basic protein is encephalitogenic in Buffalo rats. J Neuroimmunol. 1992 Apr;37(3):203-12. : https://pubmed.ncbi.nlm.nih.gov/1373154
George Deraos, et al. Citrullination of linear and cyclic altered peptide ligands from myelin basic protein (MBP(87-99)) epitope elicits a Th1 polarized response by T cells isolated from multiple sclerosis patients: implications in triggering disease. J Med Chem. 2008 Dec 25;51(24):7834-42. : https://pubmed.ncbi.nlm.nih.gov/19053745





