400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研
Ac2-26 TFA,是膜联蛋白 A1 (AnxA1) 的活性 N-末端肽,可减轻缺血再灌注诱导的急性肺损伤。
编号:124411
CAS号:151988-33-9
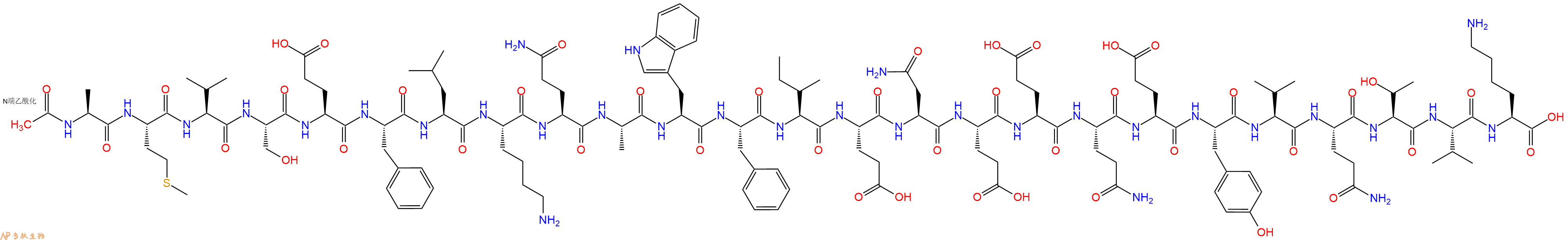
单字母:Ac-AMVSEFLKQAWFIENEEQEYVQTVK-OH
NF-κB 抑制剂。
Ac2-26,是膜联蛋白 A1 (AnxA1) 的活性 N-末端肽,可减轻缺血再灌注诱导的急性肺损伤。Ac2-26 还降低 AnxA1 蛋白表达,抑制受损肺组织中 NF-κB 和 MAPK 通路活化。
Ac2-26, an active N-terminal peptide of annexin A1 (AnxA1), attenuates ischemia-reperfusion-induced acute lung injury. Ac2-26 also decreases AnxA1 protein expression, inhibits the activation of NF-κB and MAPK pathways in the injured lung tissue.
Ac2-26是膜联蛋白A1(AnxA1)的活性N端肽。
Peptide Ac-AMVSEFLKQAWFIENEEQEYVQTVK-OH is a Research Peptide with significant interest within the field academic and medical research. Recent citations using Ac-AMVSEFLKQAWFIENEEQEYVQTVK-OH include the following: Peptides in immunoengineering JC Barrett, H Acar , MJ Mellas, MV Tirrell - Peptide applications in , 2018 - Elsevierhttps://www.sciencedirect.com/science/article/pii/B9780081007365000119 and Matthew V. Tirrell University of Chicago, Chicago, IL, United States JC Barrett, H Acar , MJ Mellas - Peptide Applications in , 2017 - books.google.comhttps://books.google.com/books?hl=en&lr=&id=OSxHDgAAQBAJ&oi=fnd&pg=PA287&dq=(%22AMVSEFLKQAWFIENEEQEYVQTVK%22+OR+%22Ac-AMVSEFLKQAWFIENEEQEYVQTVK-OH%22)+AND+peptide&ots=7AgkxTkajT&sig=R1JGApb1xdoP3cqU8FBf9SQJOqk and Matthew V. Tirrell University of Chicago, Chicago, IL, United States JC Barrett, H Acar , MJ Mellas - Peptide Applications in , 2017 - books.google.comhttps://books.google.com/books?hl=en&lr=&id=OSxHDgAAQBAJ&oi=fnd&pg=PA287&dq=(%22AMVSEFLKQAWFIENEEQEYVQTVK%22+OR+%22Ac-AMVSEFLKQAWFIENEEQEYVQTVK-OH%22)+AND+peptide&ots=7AgkxTkbhR&sig=I_O-qcbbSvPZn2r_-ORRllFpfSs Targeting and therapeutic peptides in nanomedicine for atherosclerosis EJ Chung - Experimental Biology and Medicine, 2016 - journals.sagepub.comhttps://journals.sagepub.com/doi/abs/10.1177/1535370216640940
"Annexin A1 is a phospholipid-binding protein that is involved in the regulation of inflammation. It is often found in atherosclerotic lesions and has been shown to be an anti-inflammatory cytokine. Annexin A1 (dephosphorylated) does not inhibit the activity of enzymes such as cyclooxygenase and lipoxygenase, and so it may have therapeutic potential for treatment of autoimmune diseases such as Crohns disease or bowel disease. Annexin A1 also has anti-inflammatory properties, which may be due to its ability to inhibit the production of proinflammatory cytokines such as IL-6 and TNF-α by inhibiting the activation of NFκB.\n\nAnnexin A1 (dephosphorylated) is a phospholipid-binding protein that is involved in the regulation of inflammation. It is often found in atherosclerotic lesions and has been shown to be an anti-inflammatory cytokine."
| DOI | 名称 | |
|---|---|---|
| 10.3390/ijms18081771 | Ac2-26, an Annexin A1 Peptide, Attenuates Ischemia-Reperfusion-Induced Acute Lung Injury | 下载 |





