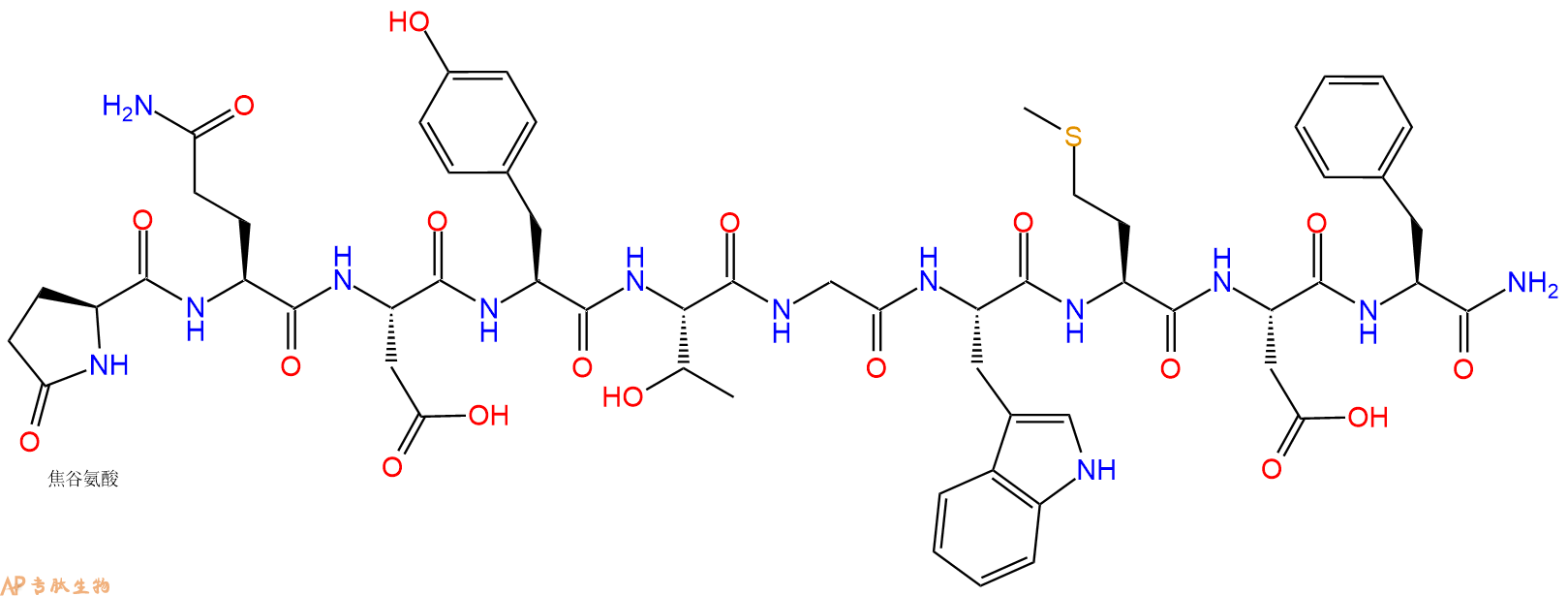
脱硫后的Caerulein。Caerulein 是一种十肽,与胃泌素和胆囊收缩素 (CCK) 具有相同的五个羧基末端氨基酸。
编号:114418
CAS号:20994-83-6
单字母:Pyr-QDYTGWMDF-NH2
| 编号: | 114418 |
| 中文名称: | 雨蛙素Caerulein (desulfated) |
| 英文名: | Caerulein (desulfated) |
| CAS号: | 20994-83-6 |
| 单字母: | Pyr-QDYTGWMDF-NH2 |
| 三字母: | Pyr 焦谷氨酸 -Gln谷氨酰胺 -Asp天冬氨酸 -Tyr酪氨酸 -Thr苏氨酸 -Gly甘氨酸 -Trp色氨酸 -Met甲硫氨酸 -Asp天冬氨酸 -Phe苯丙氨酸 -NH2C端酰胺化 |
| 氨基酸个数: | 9 |
| 分子式: | C58H73N13O18S1 |
| 平均分子量: | 1272.34 |
| 精确分子量: | 1271.49 |
| 等电点(PI): | 3.9 |
| pH=7.0时的净电荷数: | -1 |
| 平均亲水性: | -0.6 |
| 疏水性值: | -0.97 |
| 消光系数: | 6990 |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 磺酸化修饰肽 雨蛙素(Caerulein) |
Caerulein, desulfated 是脱硫后的Caerulein。Caerulein 是一种十肽,与胃泌素和胆囊收缩素 (CCK) 具有相同的五个羧基末端氨基酸。
Caerulein, desulfated is the desulfurated form of Caerulein. Caerulein is a decapeptide having the same five carboxyl-terminal amino acids as gastrin and cholecystokinin (CCK)[1].
Definition
Caerulein is a decapeptide obtained from the skin of an Australian amphibian1. It stimulates gastric, biliary and pancreatic secretion and is used as a diagnostic tool in pancreatic malfunctions1.
Discovery
Caerulein was first isolated from the skin of the frog, Hyla caerulea based on the ability of its preparation to stimulate secretion of gastric and pancreatic juices from other experimental animals1,2.
Classification
Caerulein is very similar to cholecystokinin which belongs to gastrin-type family of hormones3.
Structural Characteristics
Caerulein is a decapeptide with the sequence Pyr-Gln-Asp-Tyr(SO3H)-Thr-Gly-Trp-Met-Asp-Phe-NH23. The tyrosine sulphate residue is critical for its biological activity4. Caerulein analogs have related structure to caerulein but their functional potencies vary. Some of the analogs of caerulein include Caerulein 2.1, 3.1, 4.1 (all containing Met) and 2.2, 3.2 and 4.2 (all containing Phe)4.
Mode of action
Caerulein exerts its functions by binding to two types of receptors, CCK1 and CCK2. CCK1 receptor binding directly results in smooth muscle contraction4. Caerulein binds to CCK2 receptors on pancreatic delta cells and triggers the secretion of pancreatic juice. Specifically, it stimulates the hydrolysis of phosphatidylinositol bisphosphate to form inositol trisphosphate and diacylglycerol5. The released inositol trisphosphate could function as a second messenger to mobilize Ca2+ from an intracellular store which in turn stimulates exocytosis5.
Functions
Caerulein produces several behavioral effects in mammals such as inhibition of food and water intake, changes in mood, analgesia, sedation and antipsychotic effects6. Caerulein also stimulates the secretion of pancreatic, bile and gastric juices1.
References
1. Anastasi A, Erspamer V & Endean R (1967). Isolation and structure of caerulein, an active decapeptide from the skin of Hyla caerulea. Experientia, 23, 699-700.
2. Anastasi A, Erspamer V & Endean R (1968). Isolation and amino acid sequence of caerulein, the active decapeptide of the skin of Hyla caerulea. Arch. Biochem. Biophys., 125, 57-68.
3. Caro GD, Endean R, Erspamer V and Roseghini M (1968). Occurrence of Caerulein in extracts of the skin of Hyla caerulia and other Australian hylids. Br. J. Pharmac. Chemother, 33, 48-58.
4. Book: Handbook of Biologically active peptides by Abba J Kastin, Pg 285.
5. Roberto B, Tullio P and Claes BW (1986). Caerulein and carbamoylcholine stimulate pancreatic amylase release at resting cytosolic free Ca2+. Biochem. J., 235, 139-143.
6. Zetler G (1985). Caerulein and its analogues: neuropharmacological properties. Peptides, 6, Suppl 3, 33-46.
Johnson LR, et al. Effect of sulfation on the gastrointestinal actions of caerulein. Gastroenterology. 1970 Feb;58(2):208-16. : https://www.ncbi.nlm.nih.gov/pubmed/4904949
多肽Pyr-Gln-Asp-Tyr-Thr-Gly-Trp-Met-Asp-Phe-NH2的合成步骤:
1、合成MBHA树脂:取若干克MBHA树脂(如初始取代度为0.5mmol/g)和1倍树脂摩尔量的Fmoc-Linker-OH加入到反应器中,加入DMF,搅拌使氨基酸完全溶解。再加入树脂2倍量的DIEPA,搅拌混合均匀。再加入树脂0.95倍量的HBTU,搅拌混合均匀。反应3-4小时后,用DMF洗涤3次。用2倍树脂体积的10%乙酸酐/DMF 进行封端30分钟。然后再用DMF洗涤3次,甲醇洗涤2次,DCM洗涤2次,再用甲醇洗涤2次。真空干燥12小时以上,得到干燥的树脂{Fmoc-Linker-MHBA Resin},测定取代度。这里测得取代度为 0.3mmol/g。结构如下图:
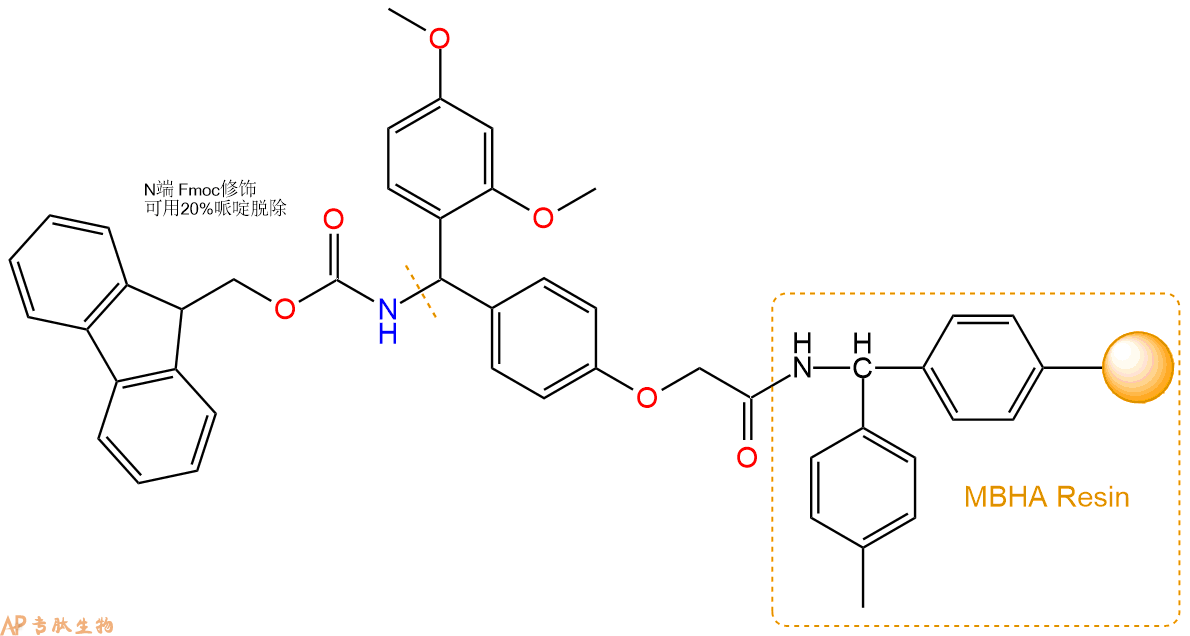
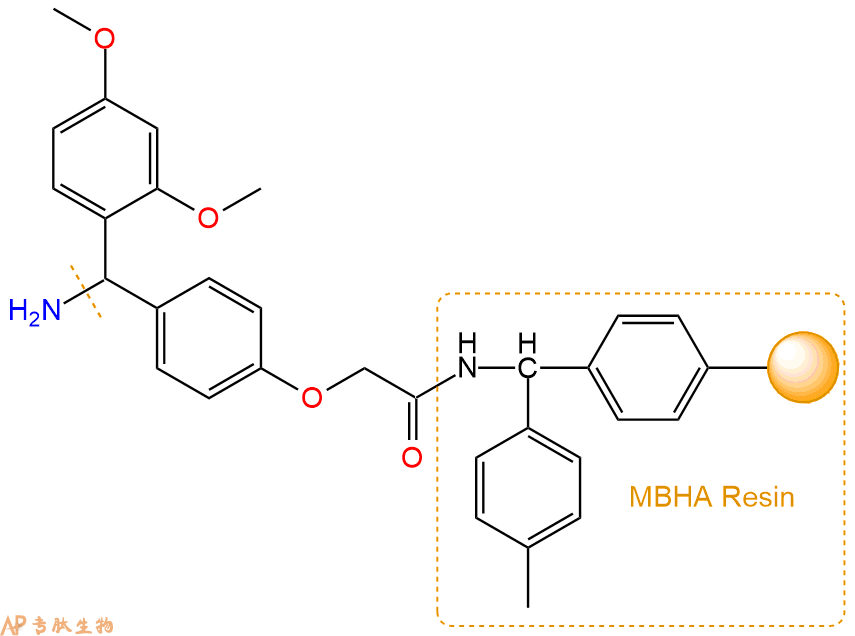
2、脱Fmoc:取2.51g的上述树脂,用DCM或DMF溶胀20分钟。用DMF洗涤2遍。加3倍树脂体积的20%Pip/DMF溶液,鼓氮气30分钟,然后2倍树脂体积的DMF 洗涤5次。得到 H2N-Linker-MBHA Resin 。(此步骤脱除Fmoc基团,茚三酮检测为蓝色,Pip为哌啶)。结构图如下:
3、缩合:取2.26mmol Fmoc-Phe-OH 氨基酸,加入到上述树脂里,加适当DMF溶解氨基酸,再依次加入4.52mmol DIPEA,2.15mmol HBTU。反应30分钟后,取小样洗涤,茚三酮检测为无色。用2倍树脂体积的DMF 洗涤3次树脂。(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。得到Fmoc-Phe-Linker-MBHA Resin。氨基酸:DIPEA:HBTU:树脂=3:6:2.85:1(摩尔比)。结构图如下:

4、依次循环步骤二、步骤三,依次得到
H2N-Phe-Linker-MBHA Resin
Fmoc-Asp(OtBu)-Phe-Linker-MBHA Resin
H2N-Asp(OtBu)-Phe-Linker-MBHA Resin
Fmoc-Met-Asp(OtBu)-Phe-Linker-MBHA Resin
H2N-Met-Asp(OtBu)-Phe-Linker-MBHA Resin
Fmoc-Trp(Boc)-Met-Asp(OtBu)-Phe-Linker-MBHA Resin
H2N-Trp(Boc)-Met-Asp(OtBu)-Phe-Linker-MBHA Resin
Fmoc-Gly-Trp(Boc)-Met-Asp(OtBu)-Phe-Linker-MBHA Resin
H2N-Gly-Trp(Boc)-Met-Asp(OtBu)-Phe-Linker-MBHA Resin
Fmoc-Thr(tBu)-Gly-Trp(Boc)-Met-Asp(OtBu)-Phe-Linker-MBHA Resin
H2N-Thr(tBu)-Gly-Trp(Boc)-Met-Asp(OtBu)-Phe-Linker-MBHA Resin
Fmoc-Tyr(tBu)-Thr(tBu)-Gly-Trp(Boc)-Met-Asp(OtBu)-Phe-Linker-MBHA Resin
H2N-Tyr(tBu)-Thr(tBu)-Gly-Trp(Boc)-Met-Asp(OtBu)-Phe-Linker-MBHA Resin
Fmoc-Asp(OtBu)-Tyr(tBu)-Thr(tBu)-Gly-Trp(Boc)-Met-Asp(OtBu)-Phe-Linker-MBHA Resin
H2N-Asp(OtBu)-Tyr(tBu)-Thr(tBu)-Gly-Trp(Boc)-Met-Asp(OtBu)-Phe-Linker-MBHA Resin
Fmoc-Gln(Trt)-Asp(OtBu)-Tyr(tBu)-Thr(tBu)-Gly-Trp(Boc)-Met-Asp(OtBu)-Phe-Linker-MBHA Resin
以上中间结构,均可在专肽生物多肽计算器-多肽结构计算器中,一键画出。
最后再经过步骤二得到 H2N-Gln(Trt)-Asp(OtBu)-Tyr(tBu)-Thr(tBu)-Gly-Trp(Boc)-Met-Asp(OtBu)-Phe-Linker-MBHA Resin,结构如下:
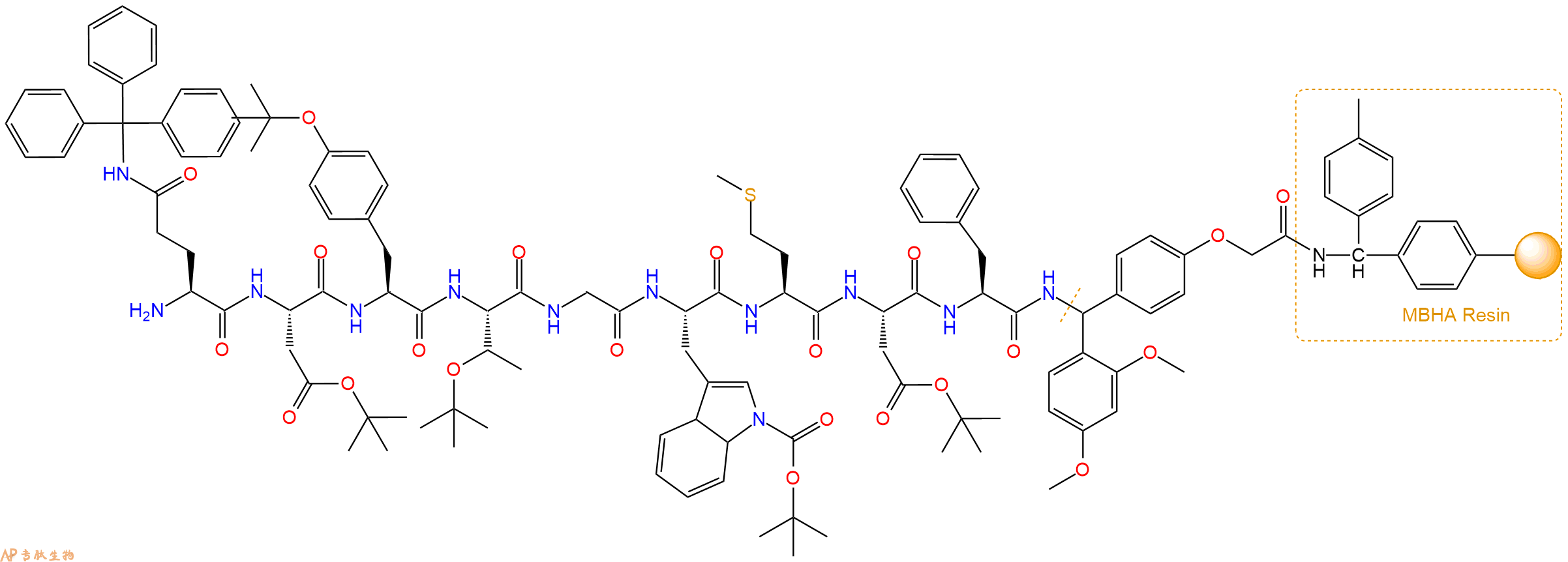
5、焦谷氨酸反应连接:在上述树脂中,加入适当DMF后,再加入2.26mmol 焦谷氨酸到树脂中,再加入4.52mmol DIPEA、2.15mmol HBTU,鼓氮气反应30分钟。用2倍树脂体积的DMF 洗涤3次树脂(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。 得到Pyr-Gln(Trt)-Asp(OtBu)-Tyr(tBu)-Thr(tBu)-Gly-Trp(Boc)-Met-Asp(OtBu)-Phe-Linker-MBHAResin。 结构如下:
5、切割:6倍树脂体积的切割液(或每1g树脂加8ml左右的切割液),摇床摇晃 2小时,过滤掉树脂,用冰无水乙醚沉淀滤液,并用冰无水乙醚洗涤沉淀物3次,最后将沉淀物放真空干燥釜中,常温干燥24小试,得到粗品Pyr-Gln-Asp-Tyr-Thr-Gly-Trp-Met-Asp-Phe-NH2。结构图见产品结构图。
切割液选择:1)TFA:H2O=95%:5%
2)TFA:H2O:TIS=95%:2.5%:2.5%
3)三氟乙酸:茴香硫醚:1,2-乙二硫醇:苯酚:水=87.5%:5%:2.5%:2.5%:2.5%
(前两种适合没有容易氧化的氨基酸,例如Trp、Cys、Met。第三种适合几乎所有的序列。)
6、纯化冻干:使用液相色谱纯化,收集目标峰液体,进行冻干,获得蓬松的粉末状固体多肽。不过这时要取小样复测下纯度 是否目标纯度。
7、最后总结:
杭州专肽生物技术有限公司(ALLPEPTIDE https://www.allpeptide.com)主营定制多肽合成业务,提供各类长肽,短肽,环肽,提供各类修饰肽,如:荧光标记修饰(CY3、CY5、CY5.5、CY7、FAM、FITC、Rhodamine B、TAMRA等),功能基团修饰肽(叠氮、炔基、DBCO、DOTA、NOTA等),同位素标记肽(N15、C13),订书肽(Stapled Peptide),脂肪酸修饰肽(Pal、Myr、Ste),磷酸化修饰肽(P-Ser、P-Thr、P-Tyr),环肽(酰胺键环肽、一对或者多对二硫键环),生物素标记肽,PEG修饰肽,甲基化修饰肽等。
以上所有内容,为专肽生物原创内容,请勿发布到其他网站上。





