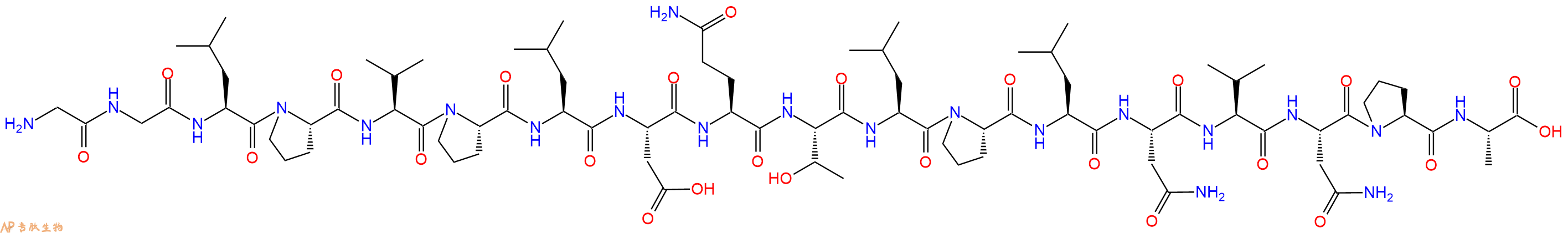
SPLUNC1 是上皮钠通道 (ENaC) 的内源性抑制剂,其负责 Na⁺ 和液体吸收穿过结肠、肾脏和气道上皮。部分序列 GGLPVPLDQTLPLNVNPA (SPLUNC1 22-39) 对应于 SPLUNC1 的 ENaC 抑制域。由于 ENaC 过度活跃有助于肺囊性纤维化的发展,该片段可能有助于开发治疗这种遗传疾病的新策略。
编号:200355
CAS号:1421132-47-9
单字母:H2N-GGLPVPLDQTLPLNVNPA-OH
| 编号: | 200355 |
| 中文名称: | SPLUNC1 (22-39) |
| CAS号: | 1421132-47-9 |
| 单字母: | H2N-GGLPVPLDQTLPLNVNPA-OH |
| 三字母: | H2N N端氨基 -Gly甘氨酸 -Gly甘氨酸 -Leu亮氨酸 -Pro脯氨酸 -Val缬氨酸 -Pro脯氨酸 -Leu亮氨酸 -Asp天冬氨酸 -Gln谷氨酰胺 -Thr苏氨酸 -Leu亮氨酸 -Pro脯氨酸 -Leu亮氨酸 -Asn天冬酰胺 -Val缬氨酸 -Asn天冬酰胺 -Pro脯氨酸 -Ala丙氨酸 -OHC端羧基 |
| 氨基酸个数: | 18 |
| 分子式: | C82H135N21O25 |
| 平均分子量: | 1815.08 |
| 精确分子量: | 1813.99 |
| 等电点(PI): | 6.26 |
| pH=7.0时的净电荷数: | -0.02 |
| 平均亲水性: | -0.625 |
| 疏水性值: | 0.19 |
| 消光系数: | - |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 抑制剂相关肽(Inhibitor Peptide) 离子通道(Ion channels)相关肽 |
SPLUNC1 is an endogenous inhibitor of the epithelial sodium channel (ENaC) which is responsible for Na⁺ and fluid absorption across colon, kidney, and airway epithelia. The partial sequence GGLPVPLDQTLPLNVNPA (SPLUNC1 22-39) corresponds to the ENaC inhibitory domain of SPLUNC1. As ENaC hyperactivity contributes to the development of cystic fibrosis in the lungs, the fragment could help to develop new strategies for the treatment of this genetic disorder.
定义
酶是用于生化反应的非常有效的催化剂。它们通过提供较低活化能的替代反应途径来加快反应速度。酶作用于底物并产生产物。一些物质降低或什至停止酶的催化活性被称为抑制剂。
发现
1965年,Umezawa H分析了微生物产生的酶抑制剂,并分离出了抑制亮肽素和抗痛药的胰蛋白酶和木瓜蛋白酶,乳糜蛋白酶抑制的胰凝乳蛋白酶,胃蛋白酶抑制素抑制胃蛋白酶,泛磷酰胺抑制唾液酸酶,乌藤酮抑制酪氨酸羟化酶,多巴汀抑制多巴胺3-羟硫基嘧啶和多巴胺3-羟色胺酶酪氨酸羟化酶和多巴胺J3-羟化酶。最近,一种替代方法已应用于预测新的抑制剂:合理的药物设计使用酶活性位点的三维结构来预测哪些分子可能是抑制剂1。已经开发了用于识别酶抑制剂的基于计算机的方法,例如分子力学和分子对接。
结构特征
已经确定了许多抑制剂的晶体结构。已经确定了三种与凝血酶复合的高效且选择性的低分子量刚性肽醛醛抑制剂的晶体结构。这三种抑制剂全部在P3位置具有一个新的内酰胺部分,而对胰蛋白酶选择性最高的两种抑制剂在P1位置具有一个与S1特异性位点结合的胍基哌啶基。凝血酶的抑制动力学从慢到快变化,而对于胰蛋白酶,抑制的动力学在所有情况下都快。根据两步机理2中稳定过渡态络合物的缓慢形成来检验动力学。
埃米尔•菲舍尔(Emil Fischer)在1894年提出,酶和底物都具有特定的互补几何形状,彼此恰好契合。这称为“锁和钥匙”模型3。丹尼尔·科什兰(Daniel Koshland)提出了诱导拟合模型,其中底物和酶是相当灵活的结构,当底物与酶4相互作用时,活性位点通过与底物的相互作用不断重塑。
在众多生物活性肽的成熟过程中,需要由其谷氨酰胺(或谷氨酰胺)前体形成N末端焦谷氨酸(pGlu)。游离形式并与底物和三种咪唑衍生抑制剂结合的人QC的结构揭示了类似于两个锌外肽酶的α/β支架,但有多个插入和缺失,特别是在活性位点区域。几种活性位点突变酶的结构分析为针对QC相关疾病5的抑制剂的合理设计提供了结构基础。
作用方式
酶是催化化学反应的蛋白质。酶与底物相互作用并将其转化为产物。抑制剂的结合可以阻止底物进入酶的活性位点和/或阻止酶催化其反应。抑制剂的种类繁多,包括:非特异性,不可逆,可逆-竞争性和非竞争性。可逆抑制剂 以非共价相互作用(例如疏水相互作用,氢键和离子键)与酶结合。非特异性抑制方法包括最终使酶的蛋白质部分变性并因此不可逆的任何物理或化学变化。特定抑制剂 对单一酶发挥作用。大多数毒药通过特异性抑制酶发挥作用。竞争性抑制剂是任何与底物的化学结构和分子几何结构非常相似的化合物。抑制剂可以在活性位点与酶相互作用,但是没有反应发生。非竞争性抑制剂是与酶相互作用但通常不在活性位点相互作用的物质。非竞争性抑制剂的净作用是改变酶的形状,从而改变活性位点,从而使底物不再能与酶相互作用而产生反应。非竞争性抑制剂通常是可逆的。不可逆抑制剂与酶形成牢固的共价键。这些抑制剂可以在活性位点附近或附近起作用。
功能
工业应用中, 酶在商业上被广泛使用,例如在洗涤剂,食品和酿造工业中。蛋白酶用于“生物”洗衣粉中,以加速蛋白质在诸如血液和鸡蛋等污渍中的分解。商业上使用酶的问题包括:它们是水溶性的,这使得它们难以回收,并且一些产物可以抑制酶的活性(反馈抑制)。
药物分子,许多药物分子都是酶抑制剂,药用酶抑制剂通常以其特异性和效力为特征。高度的特异性和效力表明该药物具有较少的副作用和较低的毒性。酶抑制剂在自然界中发现,并且也作为药理学和生物化学的一部分进行设计和生产6。
天然毒物 通常是酶抑制剂,已进化为保护植物或动物免受天敌的侵害。这些天然毒素包括一些已知最剧毒的化合物。
神经气体( 例如二异丙基氟磷酸酯(DFP))通过与丝氨酸的羟基反应生成酯,从而抑制了乙酰胆碱酯酶的活性位点。
参考
1、Scapin G (2006). Structural biology and drug discovery. Curr. Pharm. Des., 12(17):2087–2097.
2、Krishnan R, Zhang E, Hakansson K, Arni RK, Tulinsky A, Lim-Wilby MS, Levy OE, Semple JE, Brunck TK (1998). Highly selective mechanism-based thrombin inhibitors: structures of thrombin and trypsin inhibited with rigid peptidyl aldehydes. Biochemistry, 37 (35):12094-12103.
3、Fischer E (1894). Einfluss der configuration auf die wirkung der enzyme. Ber. Dt. Chem. Ges., 27:2985–2993.
4、Koshland DE (1958). Application of a theory of enzyme specificity to protein synthesis. PNAS., 44 (2):98–104.
5、Huang KF, Liu YL, Cheng WJ, Ko TP, Wang AH (2005). Crystal structures of human glutaminyl cyclase, an enzyme responsible for protein N-terminal pyroglutamate formation. PNAS., 102(37):13117-13122.
6、Holmes CF, Maynes JT, Perreault KR, Dawson JF, James MN (2002). Molecular enzymology underlying regulation of protein phosphatase-1 by natural toxins. Curr Med Chem., 9(22):1981-1989.
Definition
Enzymes are very efficient catalysts for biochemical reactions. They speed up reactions by providing an alternative reaction pathway of lower activation energy. Enzyme acts on substrate and gives rise to a product. Some substances reduce or even stop the catalytic activities of enzymes are called inhibitors.
Discovery
In 1965, Umezawa H analysed enzyme inhibitors produced by microorganisms and isolated leupeptin and antipain inhibiting trypsin and papain, chymostatin inhibiting chymotrypsin, pepstatin inhibiting pepsin, panosialin inhibiting sialidases, oudenone inhibiting tyrosine hydroxylase, dopastin inhibiting dopamine 3-hydroxylase, aquayamycin and chrothiomycin inhibiting tyrosine hydroxylase and dopamine J3-hydroxylase . Recently, an alternative approach has been applied to predict new inhibitors: rational drug design uses the three-dimensional structure of an enzyme's active site to predict which molecules might be inhibitors 1. Computer-based methods for identifying inhibitor for an enzyme have been developed, such as molecular mechanics and molecular docking.
Structural Characteristics
The crystal structures of many inhibitors have been determined. The crystal structures of three highly potent and selective low-molecular weight rigid peptidyl aldehyde inhibitors complexed with thrombin have been determined. All the three inhibitors have a novel lactam moiety at the P3 position, while the two with greatest trypsin selectivity have a guanidinopiperidyl group at the P1 position that binds in the S1 specificity site. The kinetics of inhibition vary from slow to fast with thrombin and are fast in all cases with trypsin. The kinetics are examined in terms of the slow formation of a stable transition-state complex in a two-step mechanism 2.
Emil Fischer in 1894 suggested that both the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another.This is known as "the lock and key" model 3. Daniel Koshland suggested induced fit model where substrate and enzymes are rather flexible structures, the active site is continually reshaped by interactions with the substrate as the substrate interacts with the enzyme 4.
N-terminal pyroglutamate (pGlu) formation from its glutaminyl (or glutamyl) precursor is required in the maturation of numerous bioactive peptides. The structure of human QC in free form and bound to a substrate and three imidazole-derived inhibitors reveals an alpha/beta scaffold akin to that of two-zinc exopeptidases but with several insertions and deletions, particularly in the active-site region. The structural analyses of several active-site-mutant enzymes provide a structural basis for the rational design of inhibitors against QC-associated disorders 5.
Mode of Action
Enzymes are proteins that catalyze chemical reactions. Enzymes interact with substrate and convert them into products. Inhibitor binding can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. There are a variety of types of inhibitors including: nonspecific, irreversible, reversible - competitive and noncompetitive. Reversible inhibitors bind to enzymes with non-covalent interactions like hydrophobic interactions, hydrogen bonds, and ionic bonds. Non-specific methods of inhibition include any physical or chemical changes which ultimately denature the protein portion of the enzyme and are therefore irreversible. Specific Inhibitors exert their effects upon a single enzyme. Most poisons work by specific inhibition of enzymes. A competitive inhibitor is any compound which closely resembles the chemical structure and molecular geometry of the substrate. The inhibitor may interact with the enzyme at the active site, but no reaction takes place. A noncompetitive inhibitor is a substance that interacts with the enzyme, but usually not at the active site. The net effect of a non competitive inhibitor is to change the shape of the enzyme and thus the active site, so that the substrate can no longer interact with the enzyme to give a reaction. Non competitive inhibitors are usually reversible. Irreversible Inhibitors form strong covalent bonds with an enzyme. These inhibitors may act at, near, or remote from the active site .
Functions
Industrial application, enzymes are widely used commercially, for example in the detergent, food and brewing industries. Protease enzymes are used in 'biological' washing powders to speed up the breakdown of proteins in stains like blood and egg. Problems using enzymes commercially include: they are water soluble which makes them hard to recover and some products can inhibit the enzyme activity (feedback inhibition) .
Drug molecules, many drug molecules are enzyme inhibitors and a medicinal enzyme inhibitor is usually characterized by its specificity and its potency. A high specificity and potency suggests that a drug will have fewer side effects and less toxic. Enzyme inhibitors are found in nature and are also designed and produced as part of pharmacology and biochemistry 6.
Natural poisons are often enzyme inhibitors that have evolved to defend a plant or animal against predators. These natural toxins include some of the most poisonous compounds known.
Nerve gases such as diisopropylfluorophosphate (DFP) inhibit the active site of acetylcholine esterase by reacting with the hydroxyl group of serine to make an ester.
References
Scapin G (2006). Structural biology and drug discovery. Curr. Pharm. Des., 12(17):2087–2097.
Krishnan R, Zhang E, Hakansson K, Arni RK, Tulinsky A, Lim-Wilby MS, Levy OE, Semple JE, Brunck TK (1998). Highly selective mechanism-based thrombin inhibitors: structures of thrombin and trypsin inhibited with rigid peptidyl aldehydes. Biochemistry, 37 (35):12094-12103.
Fischer E (1894). Einfluss der configuration auf die wirkung der enzyme. Ber. Dt. Chem. Ges., 27:2985–2993.
Koshland DE (1958). Application of a theory of enzyme specificity to protein synthesis. PNAS., 44 (2):98–104.
Huang KF, Liu YL, Cheng WJ, Ko TP, Wang AH (2005). Crystal structures of human glutaminyl cyclase, an enzyme responsible for protein N-terminal pyroglutamate formation. PNAS., 102(37):13117-13122.
Holmes CF, Maynes JT, Perreault KR, Dawson JF, James MN (2002). Molecular enzymology underlying regulation of protein phosphatase-1 by natural toxins. Curr Med Chem., 9(22):1981-1989.
Definition
Ion channels are membrane protein complexes and their function is to facilitate the diffusion of ions across biological membranes.
Discovery
British biophysicists Alan Hodgkin and Andrew Huxley hypothesized the existence of ion channels as a part of their Nobel Prize-winning theory of the nerve impulse, published in 1952. Existence of channel was confirmed in the 1970’s with an electrical recording technique known as the "patch clamp," by Erwin Neher and Bert Sakmann, the technique's inventors 1. 2003 Nobel Laureates Peter Agre and Roderick MacKinnon, have analysed the physico-chemical properties of ion channel functions, all of which are needed for the cell to function .
Structural Characteristics
In 1990s the first structure of an ion channel solved at atomic resolution <0.3nm is that of the bacterial porins, a family of homo-trimeric channel proteins. Each subunit contains 16 to 18 transmembrane, anti-parallel beta-strands forming a beta-barrel structure. The beta-strands are amphipathic, they contain alternating polar and non-polar residues and the inter-strand interaction is fully saturated with H-bonding. This creates a hydrophilic pore interior providing a water filled channel. The channel has a large diameter of 0.8x1.1nm, and is non-selective for small ions. However, it has an upper exclusion size limit corresponding to molecular weights of about .6 K Da. Most metabolites have molecular weights lower than .6 KDa and have been shown to pass through porin channels .
Mode of Action
An ion channel is an integral membrane protein or more typically an assembly of several proteins. Such "multi-subunit" assemblies usually involve a circular arrangement of identical or related proteins closely packed around a water-filled pore through the plane of the membrane or lipid bilayer. While large-pore channels permit the passage of ions more or less indiscriminately, the archetypal channel pore is just one or two atoms wide at its narrowest point, it conducts a specific species of ion, such as sodium or potassium, and conveys them through the membrane single file--nearly as fast as the ions move through free fluid. Access to the pore is governed by "gates," which may be opened or closed by chemical or electrical signals, or mechanical force, depending on the variety of channel. There are different groups of channels,
- Ligand gated channels neurotransmitters
- Voltage gated channels transmembrane potential (electric field)
- Second messenger gated channels nucleotides, G-proteins
- Mechanosensitive channels osmotic pressure, membrane curvature
- Gap junctions, porins not gated
X-ray analysis potassium channel from Streptomyces lividans(called K1) reveals that four identical subunits create an inverted teepee, or cone, cradling the selectivity filter of the pore in its outer end. The narrow selectivity filter is only 12? long, whereas the remainder of the pore is wider and lined with hydrophobic amino acids. A large water-filled cavity and helix dipoles are positioned so as to overcome electrostatic destabilization of an ion in the pore at the center of the bilayer. Main chain carbonyl oxygen atoms from the K1 channel signature sequence line the selectivity filter, which is held open by structural constraints to coordinate K1 ions but not smaller Na1 ions. The selectivity filter contains two K1 ions about 7.5 angstroms apart. This configuration promotes ion conduction by exploiting electrostatic repulsive forces to overcome attractive forces between K1 ions and the selectivity filter. The architecture of the pore establishes the physical principles underlying selective K1 conduction 2.
Functions
Role in nervous systems, channels are especially prominent components of the nervous system, "voltage-gated" channels conduct the nerve impulse and "transmitter-gated" channels mediate conduction across the synapses. Many toxins usually act on channels for shutting down the nervous systems of predators and prey 3.
Biological role, ion channels figure in a wide variety of biological processes that involve rapid changes in cells, such as cardiac, skeletal, and smooth muscle contraction, epithelial transport of nutrients and ions, T-cell activation and pancreatic beta-cell insulin release 4.
Drug targets, ion channels are the main targets of many drugs already used in the clinics. Most of these drugs were introduced in therapy based on the experience acquired quite empirically, and many were discovered afterward to target ion channels. Intense research is being conducted to develop new drugs acting selectively onion channel subtypes and aimed at the understanding of the intimate drug–channel interaction. Polymorphisms or mutations in ion channel genes modify sensitivity to drugs, opening the way toward the development of pharmacogenetics 5.
References
Book: Neuroscience, Ion Channels Underlying Action Potentials., by Dale P, George A, Sunderland (MA): Sinauer Associates, Inc.; 2001
2. Doyle DA, Morais CJ, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R (1998). The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science, 280(5360):69-77.
3. Camerino DC, Tricarico D, Desaphy JF (2007). Ion channel pharmacology. Neurotherapeutics, 4(2):184-98.
4. Book: Chapter 6: Electrical Excitability and Ion Channels.by Basic neurochemistry: molecular, cellular, and medical aspects., by Hille B, Catterall WA. Philadelphia: Lippincott-Raven.
5. Camerino DC, Desaphy JF, Tricarico D, Pierno S, Liantonio A (2008). Therapeutic approaches to ion channel diseases. Adv. Genet., 64:81-145.
| DOI | 名称 | |
|---|---|---|
| 10.1096/fj.12-207431 | Identification of SPLUNC1's ENaC-inhibitory domain yields novel strategies to treat sodium hyperabsorption in cystic fibrosis airways | 下载 |





