400-998-5282
专注多肽 服务科研
400-998-5282
专注多肽 服务科研
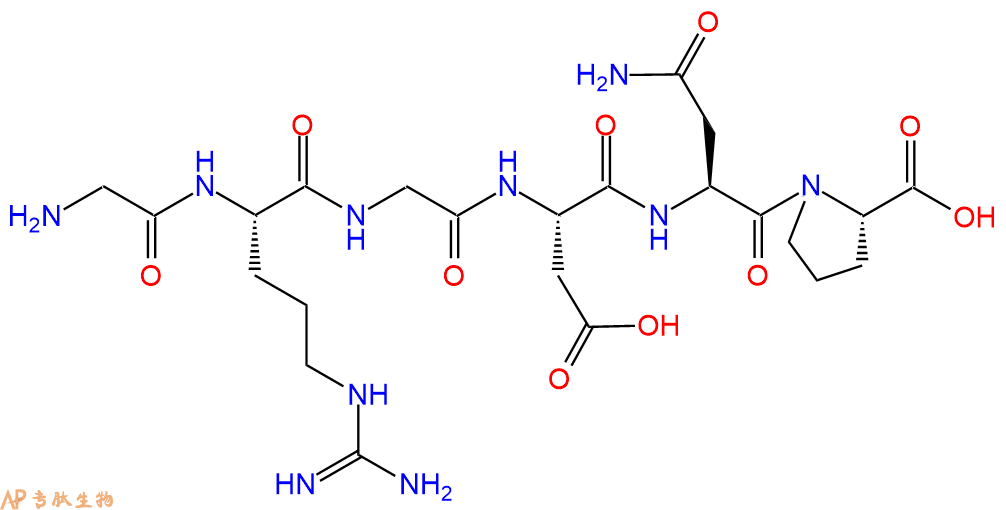
RGD peptide (GRGDNP) (TFA)作为整合素-配体相互作用的抑制剂,在细胞黏附、迁移、生长和分化中发挥重要作用。RGD peptide (GRGDNP) (TFA)通过激活构象变化促进细胞凋亡,从而增强caspase-3的活化。
编号:182326
CAS号:114681-65-1/120103-89-1
单字母:H2N-GRGDNP-OH
RGD peptide (GRGDNP) 作为整合素-配体相互作用的抑制剂,在细胞黏附、迁移、生长和分化中发挥重要作用。RGD peptide (GRGDNP) 通过激活构象变化促进细胞凋亡,从而增强caspase-3的活化。
RGD peptide (GRGDNP) acts as an inhibitor of integrin-ligand interactions and plays an important role in cell adhesion, migration, growth, and differentiation[1]. RGD peptide (GRGDNP) promote apoptosis through activation of conformation changes that enhance pro-caspase-3 activation and autoprocessing[2][3].
H-Gly-Arg-Gly-Asp-Asn-Pro-OH is a peptide that is used in the detection of damaged tissue. This peptide has been shown to have significant cytotoxicity, with an EC50 at around 100 nM. H-Gly-Arg-Gly-Asp-Asn-Pro-OH has also been shown to be reactive with integrin receptors, which are found on the surface of pluripotent cells and are involved in cell adhesion. The fluorescence intensity of this peptide increases when it binds to the integrin receptor, indicating that it may be useful for measuring changes in cellular calcium levels.
Definition
The integrins are a superfamily of cell adhesion receptors that bind to extracellular matrix EMC ligands, cell-surface ligands, and soluble ligands.
Discovery
The discovery of integrins was driven in large part by a series of early observation suggesting that adhesion to ECM is mediated at the cell surface receptors. By 1980s, it became clear that fibronectin was one of the groups of ECM proteins present in the serum that could promote the adhesion of cells to the tissue culture flask.
Structure of integrins
The integrins are a family of alpha, beta heterodimeric receptors. Integrins are expressed by all multicellular animals, but their diversity varies widely among species; for example, in mammals, 19 alpha and 8 beta subunit genes encode polypeptides that combine to form 25 different receptors, whereas the Drosophila and Caenorhabditis genomes encode only five and two integrin alpha subunits respectively.
The N-terminal portion of integrin a subunits comprises seven homologous, tandemly repeated domains of about 50 amino acids. Repeats 4-7 (or in some integrins 5-7) contain cation-binding sequences. Seven integrin a subunits (a1, a2, aE, aL, aM, aX, and aD) contain a domain of ~200 amino acids inserted between the second and third N-terminal repeats. This domain is homologous in sequence to the ‘A’ domains of von Willebrand factor, and has been shown to contain a single cation binding site1
The b ?subunit contains a region of ~240 amino acids near its N terminus that is highly conserved between different b subunits. This region may also have an A-domain-like structure with a cation binding site. The C-terminal portion of the b ?subunit contains a number of cysteine-rich repeats2.
Mechanism of action
Cell-cell and cell-substratum adhesion is mediated by the binding of integrin extracellular domains to diverse protein ligands; however, cellular control of these adhesive interactions and their translation into dynamic cellular responses, such as cell spreading or migration, requires the integrin cytoplasmic tails. These short tails bind to intracellular ligands that connect the receptors to signalling pathways and cytoskeletal networks. Hence, by binding both extracellular and intracellular ligands, integrins provide a transmembrane link for the bidirectional transmission of mechanical force and biochemical signals across the plasma membrane. One important mechanism by which cells regulate integrin function is through tight spatial and temporal control of integrin affinity for extracellular ligands. This is achieved by rapid, reversible changes in the conformation of the extracellular domains of the integrin heterodimer, so-called integrin activation3.
Functions
Proliferation: The mechanism by which integrins control proliferation involves both a direct crosstalk between integrins and growth factor receptors GFRs, and GF- independent signalling from integrins themselves. In some cells, for example, ERK signalling is induced directly by integrin adhesion, whereas the Akt pathway (which also promotes proliferation) can be activated downstream of integrins through mechanisms separate to those of GFRs4.
Apoptosis: Integrins are essential determinants of cell survival and, in many cases, prevention or alteration of integrin adhesion triggers a form of apoptosis that is known as anoikis. Anoikis is particularly relevant when cells become located in ECM environments in which they are not developmentally programmed to reside. For example, mammary epithelial cells are normally situated on a laminin-rich basement membrane but, if they are displaced to a stromal ECM of collagen I, they undergo anoikis5.
Differentiation: For some cell types, the involvement of integrins during their developmental programming to become fully mature, differentiated cells has been extensively characterized. Oligodendrocyte differentiation is a particularly neat example, because two integrin-GFR switches occur. In the first switch, PDGFaR collaborates with the vitronectin receptor avb3 integrin to promote proliferation of oligodendrocytes but, upon contact between cell processes and laminin-2, the same GFR (now in lipid rafts) works with a6b1 integrin to send survival signals. In the second switch, the EGF-family protein neuregulin sends survival and proliferation signals in oligodendrocyte precursors but, upon contact with laminin-2, the neuregulin receptors ErbB2 and ErbB4 provide signals that promote oligodendrocyte differentiation instead6.
Integrins control the cell-division axis: In studies conducted with conventional 2D tissue culture, cells divide in the plane of the dish to which they adhere, requiring alignment of the mitotic spindle parallel to the substratum (in the xy direction). Using micro-patterned ECM substrata that allow single cells to adhere with specific topologies, it has been shown that the tension exerted by the ECM (through integrin-containing adhesion) generates defined actin-cytoskeleton force fields within cells during interphase. During metaphase, cells round up to undergo mitosis, but retraction fibres transfer the polarity of tension that was established in interphase to the astral microtubules (which link the spindle poles with the cell cortex and, therefore, the plasma membrane), thereby aligning the mitotic spindle. Retraction fibres are bound to the substratum by integrins, so cell-matrix adhesion determines the internal architecture of cells, which subsequently defines the spindle position and, therefore, the division axis at mitosis7.
References
1. Michishita, M, Videm, V. and Arnaout, MA (1993). A novel divalentcation binding site in the A domain of the a integrin CR3 (CD11b/CD18) is essential for ligand binding. Cell., 72: 857-867.
2. Lee OJ, Rieu, Arnaout MA and Liddington, R. (1995a). Crystal structure of the A-domain from the b ?subunit of the integrin CR3 (CD11a/CD18). Cell., 80: 631-638.
3. Woodside DG, Liu S and Ginsberg MH (2001). Integrin activation. Thromb. Haemost., 86, 316-323.
4. Velling T, Stefansson A and Johansson S (2008). EGFR and beta1 integrins utilize different signaling pathways to activate Akt. Exp. Cell Res., 314: 309-316.
5. Pullan S, Wilson J, Metcalfe A, Edwards GM, Goberdhan N, Tilly J, Hickman JA, Dive C. and Streuli CH (1996). Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J. Cell Sci., 109: 631- 642.
6. Baron W, Colognato H and ffrench-Constant C (2005). Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia., 49: 467- 479.
7. Thery, M, Racine V, Pepin A, Piel M, Chen Y , Sibarita JB and Bornens M (2005). The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol., 7: 947-953.
| DOI | 名称 | |
|---|---|---|
| 10.1006/excr.1996.0243 | Common Principles in Cell Adhesion | 下载 |
| 10.1074/jbc.274.13.8524 | Activation of the Cdc42-associated tyrosine kinase-2 (ACK-2) by cell adhesion via integrin beta1 | 下载 |
| 10.1016/S0962-8924(97)01002-7 | Integrins, adhesion and apoptosis | 下载 |





