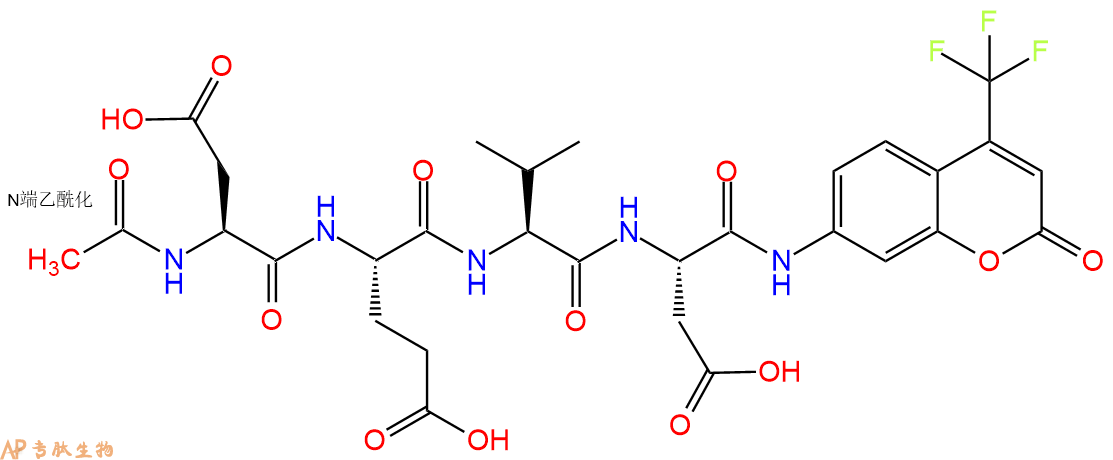
Ac-DEVD-AFC 是一种荧光底物 (λex=400 nm,λem=530 nm)。
编号:130310
CAS号:201608-14-2
单字母:Ac-DEVD-AFC
| 编号: | 130310 |
| 中文名称: | Caspase 3 (Apopain) Substrate 1f, fluorogenic (10765-05) |
| 英文名: | Caspase 3 (Apopain) Substrate 1f, fluorogenic (10765-05) |
| CAS号: | 201608-14-2 |
| 单字母: | Ac-DEVD-AFC |
| 三字母: | Ac N端乙酰化封端 -Asp天冬氨酸 -Glu谷氨酸 -Val缬氨酸 -Asp天冬氨酸 -AFC7-氨基-4-三氟甲基香豆素 |
| 氨基酸个数: | 4 |
| 分子式: | C30H34O13N4F3 |
| 平均分子量: | 715.61 |
| 精确分子量: | 715.21 |
| 等电点(PI): | - |
| pH=7.0时的净电荷数: | -3 |
| 平均亲水性: | 1.875 |
| 疏水性值: | -1.58 |
| 消光系数: | - |
| 标签: | 酶底物肽(Substrate Peptide) AFC修饰肽 细胞凋亡肽(Apoptosis Peptides) |
Ac-DEVD-AFC 是一种荧光底物 (λex=400 nm,λem=530 nm)。
Ac-DEVD-AFC is a fluorogenic substrate (λex=400 nm, λem=530 nm).
Caspase酶对应的底物,Caspases(半胱氨酸天冬氨酸蛋白酶,半胱氨酸依赖性天冬氨酸定向蛋白酶)是一类蛋白酶家族,其功能与凋亡(程序性细胞死亡),坏死和发烧(炎症)的过程密切相关。
什么是胱天蛋白酶?
胱天蛋白酶(Caspases)是含半胱氨酸的天冬氨酸蛋白水解酶,它们是为细胞凋亡的主要介质。多种受体,例如TNF-α 受体,FasL受体,TLR和死亡受体,以及Bcl-2和凋亡抑制剂(IAP)蛋白家族参与并调节该caspase依赖性凋亡途径。一旦Caspase受到上游信号(外部或内在)刺激被激活,即会参与执行下游蛋白底物的水解作用,并触发一系列事件,导致细胞分解,死亡,吞噬作用和细胞碎片的清除。
人Caspases酶
人的Caspases家族基于序列相似性和生物学功能等共性主要可分为三大类:第一类由具有长胱天蛋白酶募集结构域的“炎症”胱天蛋白酶组成,他们对P4位上的较大的芳香族或疏水性残基具有亲和力。第二类由具有短的前体结构域的“细胞凋亡效应”胱天蛋白酶组成,而第三类由具有长的前提结构域的Pap位置具有亮氨酸或缬氨酸底物亲和力的“凋亡引发剂”胱天蛋白酶组成(表1)。
表1. 人胱天蛋白酶的功能分类:
| 细胞死亡途径 | 半胱天冬酶类型 | 酵素 | 物种 |
| 细胞凋亡 | 启动器 | Caspases 2 | 人与鼠 |
| 细胞凋亡 | 启动器 | Caspases 8 | 人与鼠 |
| 细胞凋亡 | 启动器 | Caspases 9 | 人与鼠 |
| 细胞凋亡 | 启动器 | Caspases 10 | 人的 |
| 细胞凋亡 | 效应器 | Caspases 3 | 人与鼠 |
| 细胞凋亡 | 效应器 | Caspases 6 | 人与鼠 |
| 细胞凋亡 | 效应器 | Caspases 6 | 人与鼠 |
| 细胞焦亡 | 炎性的 | Caspases 1 | 人与鼠 |
| 细胞焦亡 | 炎性的 | Caspases 4 | 人的 |
| 细胞焦亡 | 炎性的 | Caspases 5 | 人的 |
启动器Caspase和效应器Caspase酶
根据其在凋亡胱天蛋白酶途径中的作用,胱天蛋白酶可分为两类:启动器和效应器Caspase酶。启动器和效应器Caspas酶都具有由小亚基和大亚基组成的催化位点,Caspase酶的识别位
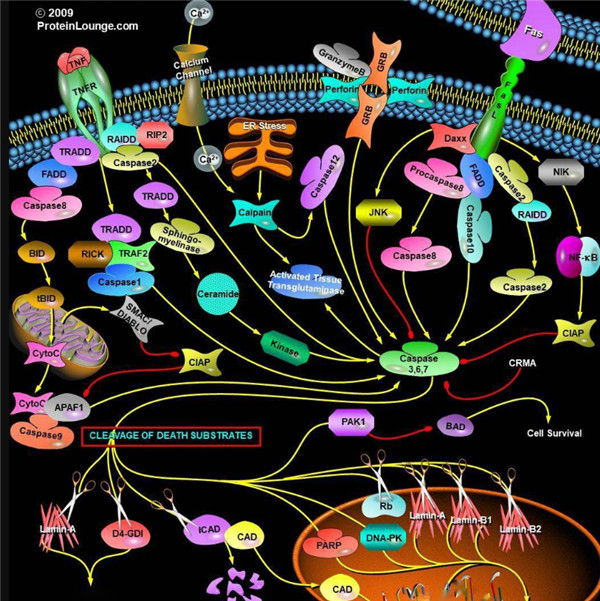
凋亡启动器Caspase酶,例如caspase-2,-8,-9和-10可以启动caspase激活级联反应。Caspase-8对于形成死亡诱导信号复合物(DISC)是必不可少的,并且在激活后,Caspase-8激活下游效应子Caspase(例如Caspase 3)并介导线粒体中细胞色素c的释放。Caspase-8已被证明对IETD肽序列具有相对较高的底物选择性。凋亡效应胱天蛋白酶例如Caspase-3,-6和-7虽然不负责启动级联途径,但是当被激活时,它们在级联的中间和后续步骤中起着不可或缺的作用。Caspase-3(CPP32 / apopain)是关键效应器,因为它放大了来自启动器Caspase的信号,使用对Caspase-3有选择性的DEVD肽序列对活化的Caspase-3进行检测,可以检测Caspase-3的活性。
Caspase酶底物和抑制剂
Caspase底物和抑制剂由两个关键成分组成:Caspase识别序列和信号产生或蛋白酶抑制基序。不同Caspase识别序列不同,一般由三个或四个氨基酸组成(表2)。Caspase酶识别序列的N端通常有乙酰基(Ac)或碳苯甲氧基(Z)基团修饰,以增强膜的通透性。对应的Caspase识别特定的肽序列为其酶促反应切割位点,释放产生信号或抑制信号的基序。Caspase的显色和荧光底物均以相似的方式起作用,其中底物的信号或颜色强度与蛋白水解活性成正比。
表2. Caspase的底物及其序列
| 多肽 | 氨基酸序列 | 对应的Caspase的种类 |
| IETD | Ile-Glu-Thr-Asp | Caspase 8,颗粒酶B |
| DEVD | Asp-Glu-Val-Asp | Caspase 3、6、7、8或10 |
| LEHD | Leu-Glu-His-Asp | Caspase 9 |
| VAD | Val-Ala-Asp | Caspase 1、2、3、6、8、9或10 |
Caspase酶的显色底物
Caspase的显色底物是有Caspase识别序列及生色基团组成,常见的生色团有pNA(对硝基苯胺或4-硝基苯胺),可使用酶标仪或分光光度计在405 nm处进行光密度检测。
表3. Caspase的显色底物
| 底物 | Caspase | 吸收(nm) | 颜色 |
| Ac-DEVD-pNA * CAS 189950-66-1 * | 半胱天冬酶3 | 405 nm | 黄色 |
| Z-DEVD-pNA | 半胱天冬酶3 | 405 nm | 黄色 |
| Z-IETD-pNA * CAS 219138-21-3 * | 半胱天冬酶8,颗粒酶B | 405 nm | 黄色 |
Caspase的荧光底物
Caspase的荧光底物的结构包含与半胱天冬酶识别相关的荧光团,例如7-氨基-4-甲基香豆素(AMC),7-氨基-4-三氟甲基香豆素(AFC), Rhodamine 110(R110)或ProRed™620。R110的Caspase底物比基于香豆素的Caspase底物(例如AMC和AFC)更敏感,但由于两步裂解过程,其动态范围更窄。 建议将R110标记的Caspase底物用于终点法测定,而将AMC和AFC标记的 Caspase底物用于动力学测定。
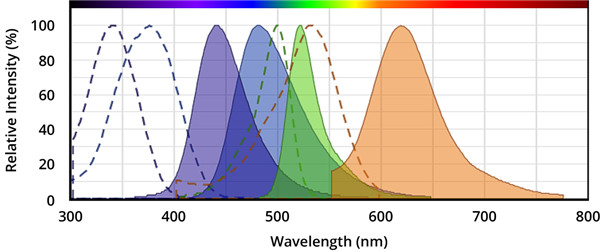
图.从左到右,分别是AMC(7-氨基-4-甲基香豆素),AFC(7-氨基-4-三氟甲基香豆素),Rhodamine 110(R110)和ProRed™620的激发和发射光谱。
表4.荧光半胱天冬酶底物。
| 底物名称 | 对应的Caspase | Ex(nm) | Em(nm) | ε¹ | Φ² |
| Ac-DEVD-AFC * CAS 201608-14-2 * | 半胱天冬酶3、7 | 376 | 482 | 17000 | 0.53 |
| Ac-DEVD-AMC * CAS 169332-61-0 * | 半胱天冬酶3、7 | 341 | 441 | 19000 | N / D |
| Z-DEVD-AFC | 半胱天冬酶3、7 | 376 | 482 | 17000 | 0.53 |
| Z-DEVD-AMC * CAS 1135416-11-3 * | 半胱天冬酶3、7 | 341 | 441 | 19000 | N / D |
| Z-DEVD-ProRed™620 | 半胱天冬酶3、7 | 532 | 619 | N / D | N / D |
| (Z-DEVD)2 -R110 * CAS 223538-61-2 * | 半胱天冬酶3、7 | 500 | 522 | 80000 | N / D |
| Z-DEVD-ProRed™620 | 半胱天冬酶3、7 | 532 | 619 | N / D | N / D |
| Ac-IETD-AFC * CAS 211990-57-7 * | 半胱天冬酶8,颗粒酶B | 376 | 482 | 17000 | 0.53 |
| Z-IETD-AFC * CAS 219138-02-0 * | 半胱天冬酶8,颗粒酶B | 376 | 482 | 17000 | 0.53 |
注意:
1.ε=在其最大吸收波长处的摩尔消光系数(单位= cm -1M -1)。
2.Φ=水性缓冲液(pH 7.2)中的荧光量子产率。
Caspase抑制剂
Caspase抑制剂能与Caspase的活性位点结合并形成可逆或不可逆的连接,通常,Caspase抑制剂的结构由Caspase识别序列,诸如醛(-CHO)或氟甲基酮(-FMK)的官能团组成。具有醛官能团的胱天蛋白酶抑制剂是可逆的,而具有FMK的抑制剂是不可逆的。半胱天冬酶底物和抑制剂都具有较小的细胞毒性作用,因此,它们是研究半胱天冬酶活性的有用工具。
表5. 可逆和不可逆的Caspase酶抑制剂
| 抑制剂 | Caspase的种类 | 是否可逆 | Ex(nm) | Em(nm) |
| Ac-DEVD-CHO * CAS 169332-60-9 * | 半胱天冬酶3、7 | 可逆的 | -- | -- |
| Ac-IETD-CHO * CAS 191338-86-0 * | 半胱天冬酶8 | 可逆的 | -- | -- |
| mFluor™450-VAD-FMK | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 406 | 445 |
| mFluor™510-VAD-FMK | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 412 | 505 |
| FITC-C6-DEVD-FMK | 半胱天冬酶3、7 | 不可逆的 | 491 | 516 |
| FITC-C6-DEVD-FMK | 半胱天冬酶3、7 | 不可逆的 | 491 | 516 |
| FITC-C6-LEHD-FMK | 半胱天冬酶9 | 不可逆的 | 491 | 516 |
| FITC-C6-LEHD-FMK | 半胱天冬酶9 | 不可逆的 | 491 | 516 |
| FAM-VAD-FMK | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 493 | 517 |
| SRB-VAD-FMK [磺胺丁胺B-VAD-FMK] | 半胱天冬酶1,2,3,6,8,9,10 | 不可逆的 | 559 | 577 |
多肽荧光标记由于没有放射性,实验操作简单。因此,目前在生物学研究中多肽荧光标记应用非常广泛,多肽荧光标记方法与荧光试剂的结构有关系,对于有游离羧基的采用的方法与接多肽反应相同,也采用HBTU/HOBt/DIEA方法连接。 在N端标记FITC的多肽需经历环化作用来形成荧光素,通常会伴有最后一个氨基酸的去除,但当有一个间隔器如氨基己酸,或者是通过非酸性环境将目的多肽从树脂上切下来时,这种情况可避免在切割的过程中被TFA切割掉。
人们利用利用荧光标记的多肽来检测目标蛋白的活性,并将 其发展的高通量活性筛选方法应用于疾病治疗靶点蛋白的药物筛选和药物开发(例如,各种激 酶、磷酸酶、肽酶等)。
专肽生物能够提供技术成熟的各种荧光标记多肽。
下面是一些常见的多肽修饰荧光物质结构:
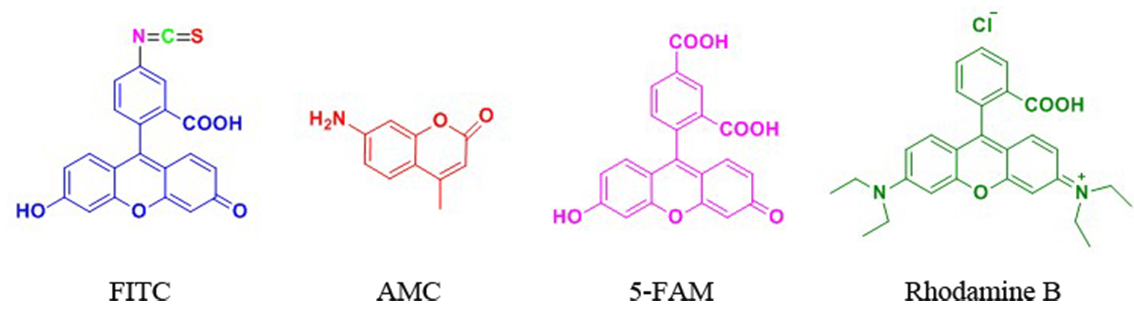
FITC标记
FITC(异硫氰酸荧光素)具有比较高的活性,我们公司可以通过两种方式将FITC标记于多肽 上:(1) 将FITC标记于赖氨酸(Lys)或被选择性地脱保护的鸟氨酸(ornithine)侧链氨基 上;(2) 将FITC标记于多肽N端氨基。
当在N端标记时,建议在最后一个氨基和由异硫氰酸酯与氨基反应产生的硫脲键之间引入 烷基间隔器(alkyl spacer),如氨基己酸(Ahx)。链接切割需要酸性环境,在N端标记FITC 的多肽需经历环化作用来形成荧光素,通常会伴有最后一个氨基酸的去除,但当有一个间隔器 如氨基己酸,或者是通过非酸性环境将目的肽从树脂上切下来时,这种情况可避免。空间位阻 被认为是在荧光染料前使用Ahx的主要原因,而不是为什么FITC不能直接偶联在多肽上的原因。
Ahx或b-Ala均可作为间隔器用于FITC标记的多肽上。
普通荧光修饰
| 荧光修饰中文名称 | N端 | N端带有linker |
| 生物素标记多肽 | Biotin- | Biotin-Ahx- |
| 异硫氰酸荧光素 | FITC- | FITC-Ahx- |
| 5-羧基荧光素 | 5-FAM- | 5-FAM-Ahx- |
| 丹磺酰荧光素 | Dansyl- | Dansyl-Ahx- |
| 5-羧基四甲基罗丹明 | TMR- (TAMRA-) | TMR-Ahx- (TAMRA-Ahx-) |
| 多肽N端 | 多肽序列中间 | N端带有linker |
| 生物素标记多肽 | Biotin- | 多肽C端 |
| Lys(Biotin)- | -Lys(Biotin)-- | -Lys(Biotin) |
| Lys(FITC)- | -Lys(FITC)- | -Lys(FITC) |
| Lys(5-FAM)- | -Lys(5-FAM)- | -Lys(5-FAM) |
| Lys(Dansyl)- | -Lys(Dansyl)- | -Lys(Dansyl) |
| Lys(TMR)- | -Lys(TMR)- | -Lys(TMR) |
| Lys(Dnp)- | -Lys(Dnp)- | -Lys(Dnp) |
专肽常做的荧光物质的激发光波长和发射光波长。可供参考选择:
| 荧光基团 | Ex(nm) | Em(nm) | 荧光基团 | Ex(nm) | Em(nm) |
| 羟基香豆素 | 325 | 386 | R-phycoerythrin (PE) (489) | 565 | 578 |
| 丹磺酰氯 | 340 | 578 | Rhodamine Red-X | 560 | 580 |
| AMC | 345 | 445 | Tamara | 565 | 580 |
| 甲氧基香豆素 | 360 | 410 | Alexa fluor 555 | 556 | 573 |
| Alexa fluor 系列 | 345 | 442 | Alexa fluor 546 | 556 | 573 |
| 氨基香豆素 | 350 | 445 | Rox | 575 | 602 |
| Dabcyl | 453 | - | Alexa fluor 568 | 578 | 603 |
| Cy2 | 490 | 510 | Texas Red | 589 | 615 |
| FAM | 495 | 517 | Alexa fluor 594 | 590 | 617 |
| Alexa fluor 488 | 494 | 517 | Alexa fluor | 621 | 639 |
| FITC | 495 | 519 | Alexa fluor 633 | 650 | 668 |
| Alexa fluor 430 | 430 | 545 | Cy5 (625) | 650 | 670 |
| 5-FAM | 492 | 518 | Alexa fluor 660 | 663 | 690 |
| Alexa fluor 532 | 530 | 530 | Cy5.5 | 675 | 694 |
| HEX | 535 | 556 | TruRed | 490; 675 | 695 |
| 5-TAMRA | 542 | 568 | Alexa fluor 680 | 679 | 702 |
| Cy3 | 550 | 570 | Cy7 | 743 | 767 |
| TRITC | 547 | 572 | Cy3.5 | 581 | 596 |
Definition
Apoptosis or programmed cell death is a normal component of the development and health of multicellular organisms. Cells die in response to a variety of stimuli and during apoptosis they do so in a controlled, regulated fashion.
Discovery
In 1885, Flemming W described the process of programmed cell death. John Kerr's discovery, in late 1960s, initially called "shrinkage necrosis" but which he later renamed "apoptosis", came about when his attention was caught by a curious form of liver cell death during his studies of acute liver injury in rats 1,2. Kerr in 1972 proposed the term apoptosis is for mechanism of controlled cell deletion, which appears to play a complementary but opposite role to mitosis in the regulation of animal cell populations. Its morphological features suggest that it is an active, inherently programmed phenomenon, and it has been shown that it can be initiated or inhibited by a variety of environmental stimuli, both physiological and pathological 3.
Structural Characteristics
Heterodimerization between members of the Bcl-2 family of proteins is a key event in the regulation of programmed cell death. The molecular basis for heterodimer formation was investigated by determination of the solution structure of a complex between the survival protein Bcl-xL and the death-promoting region of the Bcl-2-related protein Bak. The structure and binding affinities of mutant Bak peptides indicate that the Bak peptide adopts an amphipathic helix that interacts with Bcl-xL through hydrophobic and electrostatic interactions. Mutations in full-length Bak that disrupt either type of interaction inhibit the ability of Bak to heterodimerize with Bcl-xL 4.
The structure of the 16–amino acid peptide complexed with a biologically active deletion mutant of Bcl-xL was determined by nuclear magnetic resonance spectroscopy (NMR). The structure was determined from a total of 2813 NMR-derived restraints and is well defined by the NMR data. The Bak peptide forms a helix when complexed to Bcl-xL. The COOH terminal portion of the Bak peptide interacts predominantly with residues in the BH2 and BH3 regions. Melanoma inhibitor of apoptosis (ML-IAP) is a potent anti-apoptotic protein that is upregulated in a number of melanoma cell lines but not expressed in most normal adult tissues. Overexpression of IAP proteins, such as ML-IAP or the ubiquitously expressed X-chromosome-linked IAP (XIAP), in human cancers has been shown to suppress apoptosis induced by a variety of stimuli. X-ray crystal structures of ML-IAP-BIR in complex with Smac- and phage-derived peptides, together with peptide structure−activity-relationship data, indicate that the peptides can be modified to provide increased binding affinity and selectivity for ML-IAP-BIR relative to XIAP-BIR3 5.
Mode of Action
Upon receiving specific signals instructing the cells to undergo apoptosis a number of distinctive changes occur in the cell. Families of proteins known as caspases are typically activated in the early stages of apoptosis. These proteins breakdown or cleave key cellular components that are required for normal cellular function including structural proteins in the cytoskeleton and nuclear proteins such as DNA repair enzymes. The caspases can also activate other degradative enzymes such as DNases, which begin to cleave the DNA in the nucleus.
Apoptotic cells display distinctive morphology during the apoptotic process. Typically, the cell begins to shrink following the cleavage of lamins and actin filaments in the cytoskeleton. The breakdown of chromatin in the nucleus often leads to nuclear condensation and in many cases the nuclei of apoptotic cells take on a "horse-shoe" like appearance. Cells continue to shrink, packaging themselves into a form that allows for their removal by macrophages. There are a number of mechanisms through which apoptosis can be induced in cells. The sensitivity of cells to any of these stimuli can vary depending on a number of factors such as the expression of pro- and anti-apoptotic proteins (eg. the Bcl-2 proteins or the Inhibitor of Apoptosis Proteins), the severity of the stimulus and the stage of the cell cycle. The Bcl-2 family of proteins plays a central role in the regulation of apoptotic cell death induced by a wide variety of stimuli. Some proteins within this family, including Bcl-2 and Bcl-xL, inhibit programmed cell death, and others, such as Bax and Bak, can promote apoptosis 6, 7.
Functions
For development, Apoptosis is as needed for proper development as mitosis is. Examples: The resorption of the tadpole tail at the time of its metamorphosis into a frog occurs by apoptosis.
Integrity of the organism, Apoptosis is needed to destroy cells that represent a threat to the integrity of the organism. Examples: Cells infected with viruses8.
Cells of the immune system, as cell-mediated immune responses wane, the effector cells must be removed to prevent them from attacking body constituents. CTLs induce apoptosis in each other and even in themselves 9.
Cells with DNA damage, damage to its genome can cause a cell to disrupt proper embryonic development leading to birth defects to become cancerous.
References
1. Kerr JF (1965). A histochemical study of hypertrophy and ischaemic injury of rat liver with special reference to changes in lysosomes. Journal of Pathology and Bacteriology, 90(90):419-435.
2. Kerr JF, Wyllie AH, Currie AR (1972). Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer., 26(4):239-257.
3. O'Rourke MG, Ellem KA (2000). John Kerr and apoptosis. Med. J. Aust., 173(11-12): 616-617.
4. Franklin MC, Kadkhodayan S, Ackerly H, Alexandru D, Distefano MD, Elliott LO, Flygare JA, Mausisa G, Okawa DC, Ong D, Vucic D, Deshayes K, Fairbrother WJ (2003). Structure and function analysis of peptide antagonists of melanoma inhibitor of apoptosis (ML-IAP). Biochemistry, 42(27):8223-8231.
5. Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW (1997). Structure of bcl-xl-bak peptide complex: recognition between regulators of apoptosis. Science, 275(5302):983-986.
6. Hanada M, Aimé-Sempé C, Sato T, Reed JC (1995). Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J. Biol. Chem., 270(20):11962-11969.
7. Cheng EHY, Levine B, Boise LH, Thompson CB, Hardwic JM (1996). Bax-independent inhibition of apoptosis by Bcl-xL.Nature, 379:554-556.
8. Alimonti JB, Ball TB, Fowke KR (2003). Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J Gen Virology., 84(84): 1649-1661.
9. Werlen G, Hausmann B, Naeher D, Palmer E (2003). Signaling life and death in the thymus: timing is everything. Science. 299(5614):1859-1863.
| DOI | 名称 | |
|---|---|---|
| 10.1038/sj.cdd.4400928 | Nuclear localization of DEDD leads to caspase-6 activation through its death effector domain and inhibition of RNA polymerase I dependent transcription | 下载 |
| 10.1038/sj.cdd.4401247 | Inhibition of papain-like cysteine proteases and legumain by caspase-specific inhibitors: when reaction mechanism is more important than specificity | 下载 |
| 10.1074/jbc.M308347200 | Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins | 下载 |
| 10.1111/j.1365-3083.2011.02589.x | Lysosomal membrane permeabilization induces cell death in human mast cells | 下载 |
| 10.1074/jbc.M305110200 | Human caspase-7 activity and regulation by its N-terminal peptide | 下载 |
| 10.1038/sj.emboj.7600210 | Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes | 下载 |
| 10.1002/dvdy.20124 | Activation of apoptosis and caspase-3 in zebrafish early gastrulae | 下载 |
| 10.1074/jbc.M410915200 | Molecular ordering of the caspase activation cascade initiated by the cytotoxic T lymphocyte/natural killer (CTL/NK) protease granzyme B | 下载 |
| 10.1016/j.febslet.2005.03.002 | Sensitization of stefin B-deficient thymocytes towards staurosporin-induced apoptosis is independent of cysteine cathepsins | 下载 |
| 10.2353/ajpath.2006.050323 | Anti-apoptotic function of gelsolin in fas antibody-induced liver failure in vivo | 下载 |
| 10.1016/j.neuro.2006.06.006 | Proteasome inhibitor MG-132 induces dopaminergic degeneration in cell culture and animal models | 下载 |
| 10.1016/j.febslet.2007.10.005 | Cysteine cathepsins are not involved in Fas/CD95 signalling in primary skin fibroblasts | 下载 |
| 10.1016/j.febslet.2007.12.002 | Similar toxicity of the oligomeric molten globule state and the prefibrillar oligomers | 下载 |
| 10.1016/j.ejphar.2008.11.008 | Azaphenylalanine-based serine protease inhibitors induce caspase-mediated apoptosis | 下载 |
| 10.1074/jbc.M807913200 | Nucleophosmin is cleaved and inactivated by the cytotoxic granule protease granzyme M during natural killer cell-mediated killing | 下载 |
| 10.1111/j.1365-2362.2009.02133.x | Keratin 18 provides resistance to Fas-mediated liver failure in mice | 下载 |
| 10.1016/j.bbapap.2009.04.011 | Cysteine cathepsins are not critical for TNF-alpha-induced cell death in T98G and U937 cells | 下载 |
| 10.1016/j.immuni.2009.05.007 | Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases | 下载 |
| 10.1016/j.chembiol.2010.07.011 | Selective and sensitive monitoring of caspase-1 activity by a novel bioluminescent activity-based probe | 下载 |
| 10.1186/1479-5876-9-123 | Liver mitochondrial dysfunction is reverted by insulin-like growth factor II (IGF-II) in aging rats | 下载 |
| 10.1021/jm300881a | Synthesis and characterization of a novel prostate cancer-targeted phosphatidylinositol-3-kinase inhibitor prodrug | 下载 |
| 10.1371/journal.pone.0044694 | Viral cross-class serpin inhibits vascular inflammation and T lymphocyte fratricide; a study in rodent models in vivo and human cell lines in vitro | 下载 |
| 10.1016/j.bbamcr.2013.05.007 | N-terminally truncated forms of human cathepsin F accumulate in aggresome-like inclusions | 下载 |
| 10.1038/onc.2013.314 | Stefin B deficiency reduces tumor growth via sensitization of tumor cells to oxidative stress in a breast cancer model | 下载 |
| 10.1039/c4dt00463a | Structural characterization and biological evaluation of a clioquinol-ruthenium complex with copper-independent antileukaemic activity | 下载 |
| 10.1016/j.mrgentox.2014.07.002 | Evaluation of potential antigenotoxic, cytotoxic and proapoptotic effects of the olive oil by-product "alperujo", hydroxytyrosol, tyrosol and verbascoside | 下载 |
| 10.1073/pnas.93.25.14559 | BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases | 下载 |
| 10.1006/bbrc.1997.7370 | Recombinant caspase-3 expressed in Pichia pastoris is fully activated and kinetically indistinguishable from the native enzyme | 下载 |
| 10.1084/jem.191.1.33 | The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore | 下载 |
| 10.1016/j.lfs.2006.12.013 | Effect of testosterone on oxidative stress and cell damage induced by 3-nitropropionic acid in striatum of ovariectomized rats | 下载 |
| 10.1111/j.1349-7006.2008.00868.x | Differential responsiveness of human hepatoma cells versus normal hepatocytes to TRAIL in combination with either histone deacetylase inhibitors or conventional cytostatics | 下载 |
| 10.1016/j.cbi.2009.06.003 | N-acetylcysteine, coenzyme Q10 and superoxide dismutase mimetic prevent mitochondrial cell dysfunction and cell death induced by d-galactosamine in primary culture of human hepatocytes | 下载 |
| 10.1002/2211-5463.12044 | Production of biologically active IL-36 family cytokines through insertion of N-terminal caspase cleavage motifs | 下载 |
| 10.1016/j.neuropharm.2016.11.019 | Inhibition of cathepsin X reduces the strength of microglial-mediated neuroinflammation | 下载 |
多肽Ac-Asp-Glu-Val-Asp-AFC的合成步骤:
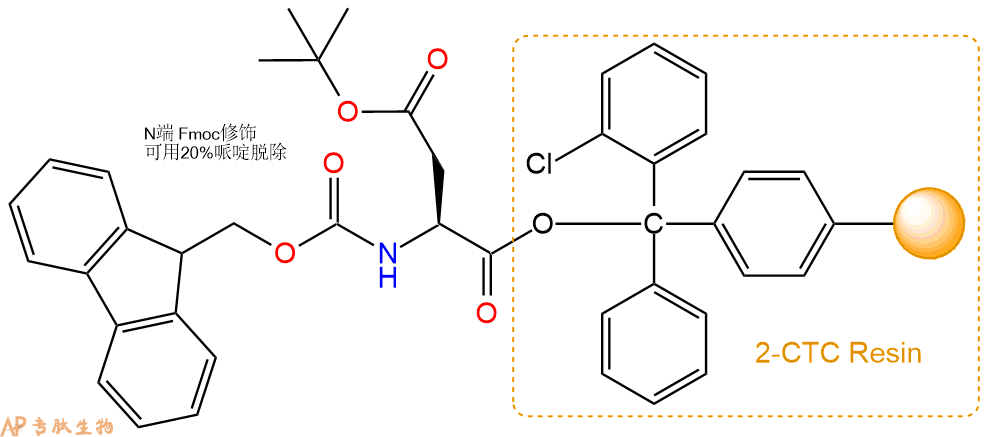
1、合成CTC树脂:称取1.41g CTC Resin(如初始取代度约为0.43mmol/g)和0.73mmol Fmoc-Asp(OtBu)-OH于反应器中,加入适量DCM溶解氨基酸(需要注意,此时CTC树脂体积会增大好几倍,避免DCM溶液过少),再加入1.82mmol DIPEA(Mw:129.1,d:0.740g/ml),反应2-3小时后,可不抽滤溶液,直接加入1ml的HPLC级甲醇,封端半小时。依次用DMF洗涤2次,甲醇洗涤1次,DCM洗涤一次,甲醇洗涤一次,DCM洗涤一次,DMF洗涤2次(这里使用甲醇和DCM交替洗涤,是为了更好地去除其他溶质,有利于后续反应)。得到 Fmoc-Asp(OtBu)-CTC Resin。结构图如下:
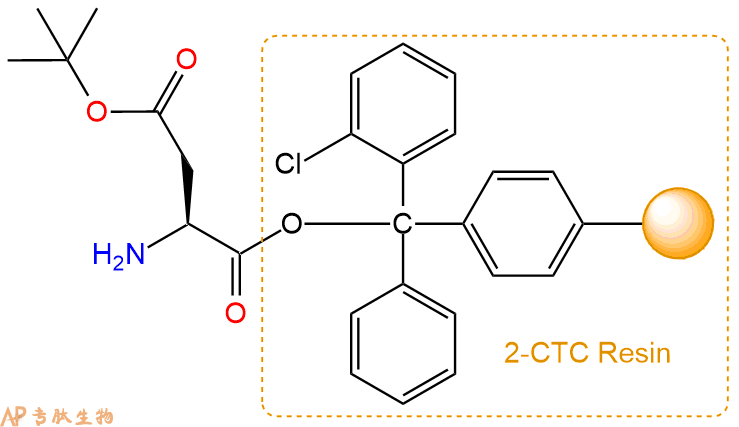
2、脱Fmoc:加3倍树脂体积的20%Pip/DMF溶液,鼓氮气30分钟,然后2倍树脂体积的DMF 洗涤5次。得到 H2N-Asp(OtBu)-CTC Resin 。(此步骤脱除Fmoc基团,茚三酮检测为蓝色,Pip为哌啶)。结构图如下:
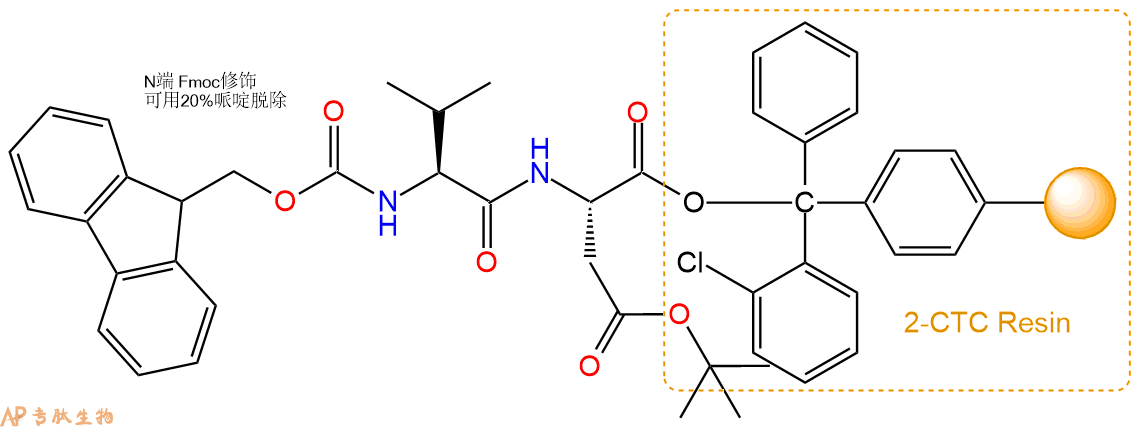
3、缩合:取1.82mmol Fmoc-Val-OH 氨基酸,加入到上述树脂里,加适当DMF溶解氨基酸,再依次加入3.64mmol DIPEA,1.73mmol HBTU。反应30分钟后,取小样洗涤,茚三酮检测为无色。用2倍树脂体积的DMF 洗涤3次树脂。(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。得到Fmoc-Val-Asp(OtBu)-CTC Resin。氨基酸:DIPEA:HBTU:树脂=3:6:2.85:1(摩尔比)。结构图如下:
4、依次循环步骤二、步骤三,依次得到
H2N-Val-Asp(OtBu)-CTC Resin
Fmoc-Glu(OtBu)-Val-Asp(OtBu)-CTC Resin
H2N-Glu(OtBu)-Val-Asp(OtBu)-CTC Resin
Fmoc-Asp(OtBu)-Glu(OtBu)-Val-Asp(OtBu)-CTC Resin
以上中间结构,均可在专肽生物多肽计算器-多肽结构计算器中,一键画出。
最后再经过步骤二得到 H2N-Asp(OtBu)-Glu(OtBu)-Val-Asp(OtBu)-CTC Resin,结构如下:
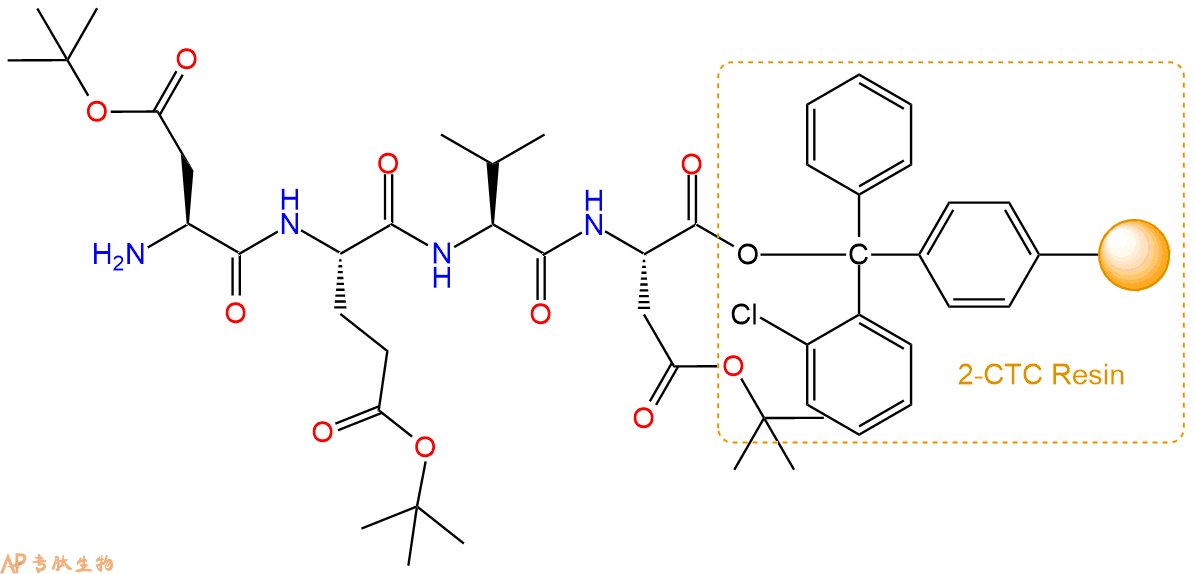
5、乙酸酐反应连接:在上述树脂中,加入适当DMF后,再加入1.82mmol 乙酸酐到树脂中,再加入3.64mmol DIPEA、1.73mmol HBTU,鼓氮气反应30分钟。用2倍树脂体积的DMF 洗涤3次树脂(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。 得到Ac-Asp(OtBu)-Glu(OtBu)-Val-Asp(OtBu)-CTC Resin。 结构如下:
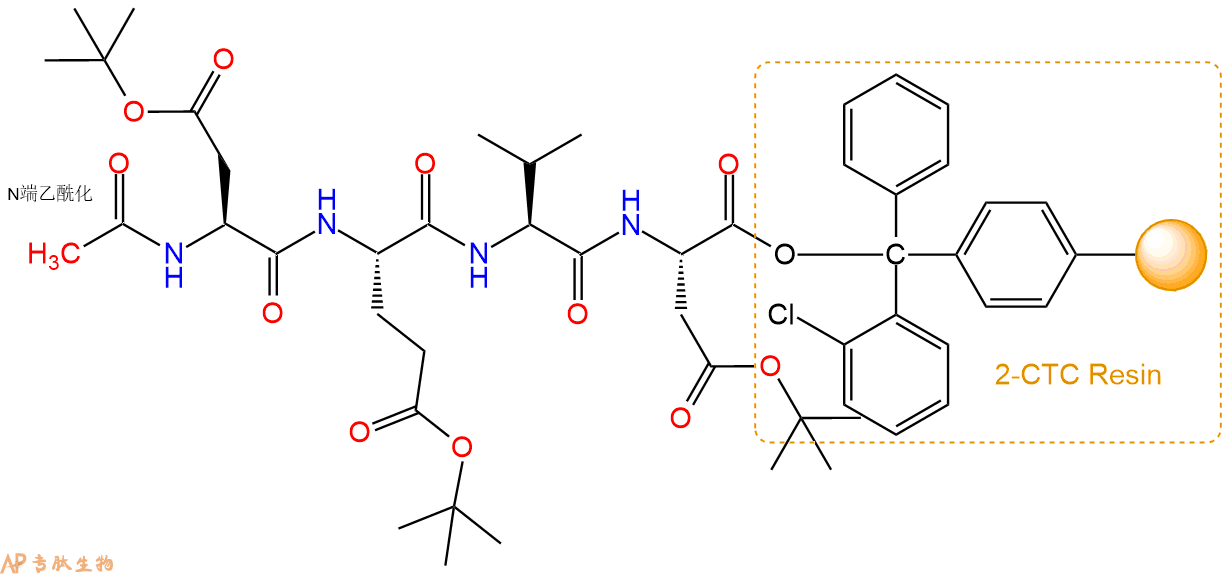
6、全保护切割:配置0.5%TFA/DCM溶液,溶液体积约为树脂体积的3倍。再次用DCM洗涤树脂2遍(去除残留DMF),后将配置好的溶液倒入到反应器中,反应30分钟。抽滤树脂,收集滤液(此时多肽已经从树脂上分离,存在于滤液中)。多肽序列为 Ac-Asp(OtBu)-Glu(OtBu)-Val-Asp(OtBu)-CTC Resin。 在滤液中添加DIEPA,调PH至7-8。用饱和NaHCO3洗涤滤液,分离出DCM层溶液。可适当旋蒸DCM层溶液,减少有机溶剂。再次加入1或2倍体积的乙酸乙酯,用稀HCl溶液调PH至微酸性,将多肽从DCM层萃取到乙酸乙酯层。用饱和NaCl洗涤2次乙酸乙酯层。用无水硫酸镁吸收乙酸乙酯层的水分。通过减压旋蒸,直接将乙酸乙酯完全旋蒸掉,得到晶体状固体多肽,用于下一步C端反应。或通过减压旋蒸保留适量乙酸乙酯的溶液体积,加入冰乙醚析出 多肽,然后对多肽进行烘干操作即可用于下一步C端反应。Ac-Asp(OtBu)-Glu(OtBu)-Val-Asp(OtBu)-COOH的结构图如下。
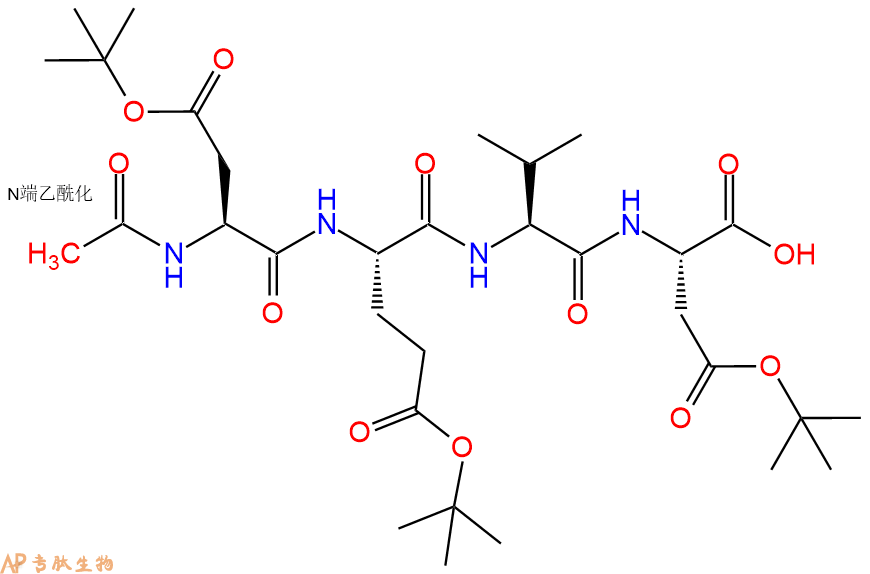
7、7-氨基-4-三氟甲基香豆素反应连接:在上述树脂中,加入适当DMF后,再加入1.82mmol 7-氨基-4-三氟甲基香豆素到树脂中,再加入3.64mmol DIPEA、1.73mmol HBTU,鼓氮气反应30分钟。用2倍树脂体积的DMF 洗涤3次树脂(洗涤树脂,去掉残留溶剂,为下一步反应做准备)。 得到 Ac-Asp(OtBu)-Glu(OtBu)-Val-Asp(OtBu)-AFC。 结构如下:
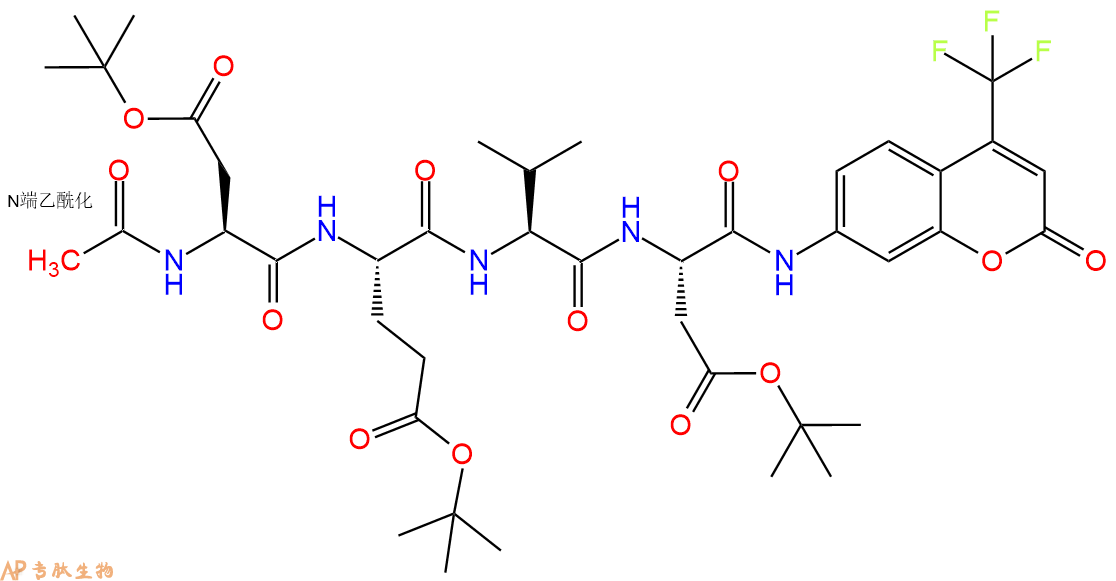
8、切割:6倍树脂体积的切割液(或每1g树脂加8ml左右的切割液),摇床摇晃 2小时,过滤掉树脂,用冰无水乙醚沉淀滤液,并用冰无水乙醚洗涤沉淀物3次,最后将沉淀物放真空干燥釜中,常温干燥24小试,得到粗品Ac-Asp-Glu-Val-Asp-AFC。结构图见产品结构图。
切割液选择:1)TFA:H2O=95%:5%、TFA:H2O=97.5%:2.5%
2)TFA:H2O:TIS=95%:2.5%:2.5%
3)三氟乙酸:茴香硫醚:1,2-乙二硫醇:苯酚:水=87.5%:5%:2.5%:2.5%:2.5%
(前两种适合没有容易氧化的氨基酸,例如Trp、Cys、Met。第三种适合几乎所有的序列。)
9、纯化冻干:使用液相色谱纯化,收集目标峰液体,进行冻干,获得蓬松的粉末状固体多肽。不过这时要取小样复测下纯度 是否目标纯度。
10、最后总结:
杭州专肽生物技术有限公司(ALLPEPTIDE https://www.allpeptide.com)主营定制多肽合成业务,提供各类长肽,短肽,环肽,提供各类修饰肽,如:荧光标记修饰(CY3、CY5、CY5.5、CY7、FAM、FITC、Rhodamine B、TAMRA等),功能基团修饰肽(叠氮、炔基、DBCO、DOTA、NOTA等),同位素标记肽(N15、C13),订书肽(Stapled Peptide),脂肪酸修饰肽(Pal、Myr、Ste),磷酸化修饰肽(P-Ser、P-Thr、P-Tyr),环肽(酰胺键环肽、一对或者多对二硫键环),生物素标记肽,PEG修饰肽,甲基化修饰肽
以上所有内容,为专肽生物原创内容,请勿发布到其他网站上。





