首先从澳大利亚蛙蓝斑蛙的皮肤中分离出的这种硫酸化肽是一种比其类似物CCK-8更有效的促分泌剂。
编号:161723
CAS号:17650-98-5
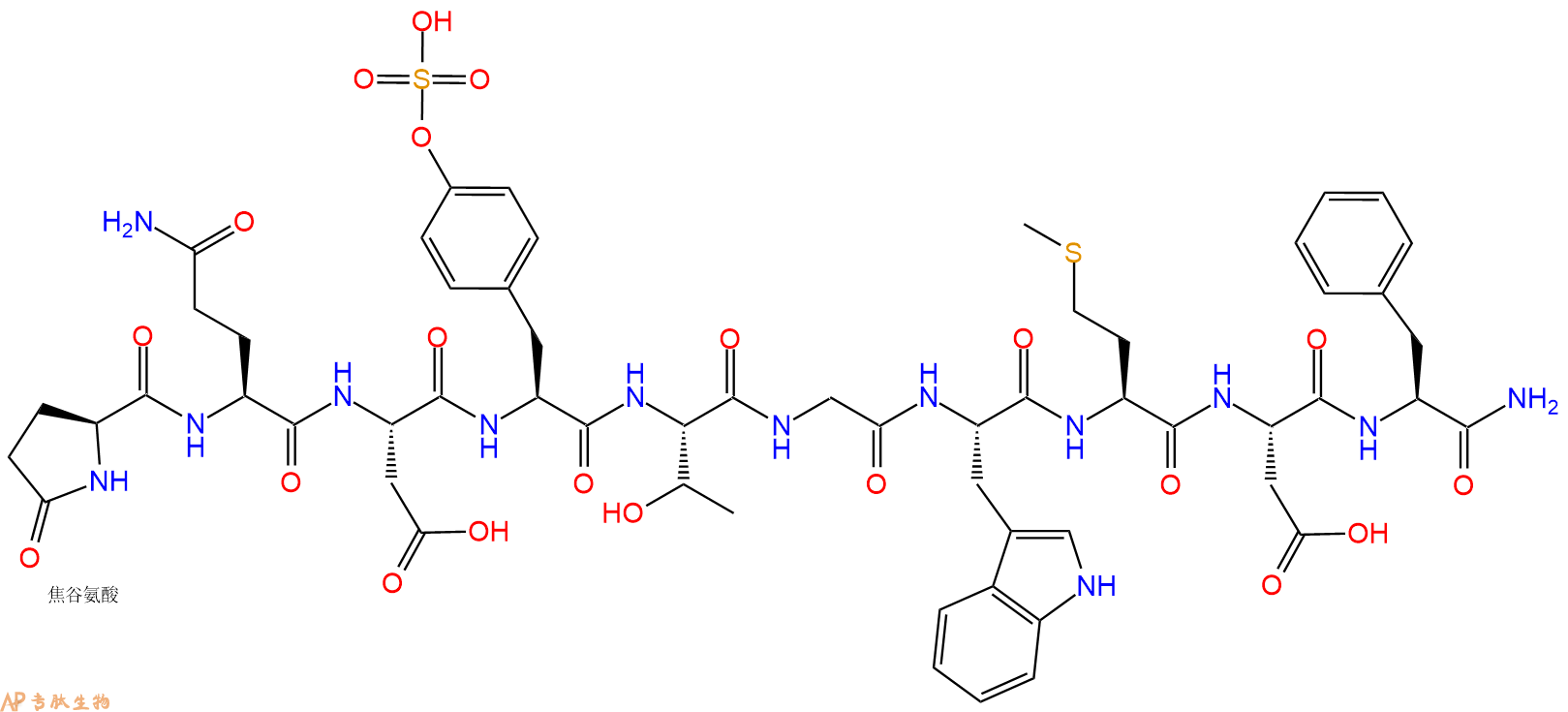
单字母:Pyr-QD-sTyr-TGWMDF-NH2
| 编号: | 161723 |
| 中文名称: | 雨蛙素Caerulein |
| 英文名: | Caerulein |
| CAS号: | 17650-98-5 |
| 单字母: | Pyr-QD-sTyr-TGWMDF-NH2 |
| 三字母: | Pyr 焦谷氨酸 -Gln谷氨酰胺 -Asp天冬氨酸 -Tyr(SO3H)磺酸化酪氨酸 -Thr苏氨酸 -Gly甘氨酸 -Trp色氨酸 -Met甲硫氨酸 -Asp天冬氨酸 -Phe苯丙氨酸 -NH2C端酰胺化 |
| 氨基酸个数: | 9 |
| 分子式: | C58H73N13O21S2 |
| 平均分子量: | 1352.4 |
| 精确分子量: | 1351.45 |
| 等电点(PI): | - |
| pH=7.0时的净电荷数: | -2 |
| 平均亲水性: | -0.2 |
| 疏水性值: | -0.98 |
| 消光系数: | 5500 |
| 来源: | 人工化学合成,仅限科学研究使用,不得用于人体。 |
| 储存条件: | 负80℃至负20℃ |
| 标签: | 磺酸化修饰肽 胆囊收缩素(Cholecystokinin) 雨蛙素(Caerulein) |
This sulfated peptide isolated first from the skin of the Australian frog Litoria caerulea is a more potent secretagogue than its analog CCK-8. Ceruletide is applied in the diagnosis of pancreatic function and, in high dosage, for the induction of pancreatitis in experimental animals.
Definition
Cholecystokinin (CCK), also called pancreozymin, is a peptide hormone in the small intestine that constitutes the classical gut hormone triad together with gastrin and secretin1. CCK is secreted into the blood following ingestion of a meal and plays a critical role in the ingestion, absorption, intestinal motility, satiety signaling, inhibition of gastric acid secretion and digestion of food1.
Discovery
CCK was discovered in 1928 because of its ability to induce gallbladder contraction2.
Classification
CCK is a neuropeptide. It is a family of hormones identified by the number of amino acids, for eg: CCK58 and CCK331.
Structural Characteristics
Prepro-CCK is a115 amino acid peptide that is first cleaved to pro-CCK which in turn results in CCK58, the major processed form of CCK3. CCK58 assumes a helix-turn-helix configuration3.
Mode of action
CCK binds to CCK receptors on the cell membrane that when activated increase the turnover of phosphatidyl inositol which results in the release of intracellular calcium4. The calcium released causes increased enzyme secretion either directly or through activation of protein kinase C4.
Functions
CCK induces the gall bladder to contract and eject bile into the intestine5. It stimulates the acinar cells of the pancreas to release water and ions and stimulates the secretion of a juice rich in pancreatic digestive enzymes5. It is known to induce growth of the exocrine pancreas and to stimulate insulin secretion5. CCK is the most abundant neuropeptide in the human brain where it induces panic attacks that are antagonized by a central cholecystokinin receptor antagonist6. ProCCK is expressed in certain neuroendocrine tumors and sarcomas, and the secretion of CCK is impaired in celiac disease and bulimia nervosa7.
References
1. Fink H, Rex A, Voits M, Voigt JP (1998). Major biological actions of CCK--a critical evaluation of research findings. Exp Brain Res., 123 (1-2), 77–83.
2. Hunt, J. N. (1948). A method for estimating peptic activity in gastric contents. Biochem. J., 42, 104-109.
3. Book: Neuropeptides By Fleur L. Strand, 387-389.
4. Dufresne M, Seva C, Fourmy D (2006). Cholecystokinin and gastrin receptors. Physiol. Rev., 86 (3), 805–47.
5. Chandra R, Liddle RA (2007). Cholecystokinin. Curr Opin Endocrinol Diabetes Obes., 14(1), 63-7.
6. Rehfeld JF, Friis-Hansen L, Goetze JP, Hansen TV (2007). The biology of cholecystokinin and gastrin peptides. Curr Top Med Chem, 7(12), 1154-65.
7. Rehfeld JF (2004). Clinical endocrinology and metabolism. Cholecystokinin. Best Pract Res Clin Endocrinol Metab., 18(4), 569-86.
Definition
Caerulein is a decapeptide obtained from the skin of an Australian amphibian1. It stimulates gastric, biliary and pancreatic secretion and is used as a diagnostic tool in pancreatic malfunctions1.
Discovery
Caerulein was first isolated from the skin of the frog, Hyla caerulea based on the ability of its preparation to stimulate secretion of gastric and pancreatic juices from other experimental animals1,2.
Classification
Caerulein is very similar to cholecystokinin which belongs to gastrin-type family of hormones3.
Structural Characteristics
Caerulein is a decapeptide with the sequence Pyr-Gln-Asp-Tyr(SO3H)-Thr-Gly-Trp-Met-Asp-Phe-NH23. The tyrosine sulphate residue is critical for its biological activity4. Caerulein analogs have related structure to caerulein but their functional potencies vary. Some of the analogs of caerulein include Caerulein 2.1, 3.1, 4.1 (all containing Met) and 2.2, 3.2 and 4.2 (all containing Phe)4.
Mode of action
Caerulein exerts its functions by binding to two types of receptors, CCK1 and CCK2. CCK1 receptor binding directly results in smooth muscle contraction4. Caerulein binds to CCK2 receptors on pancreatic delta cells and triggers the secretion of pancreatic juice. Specifically, it stimulates the hydrolysis of phosphatidylinositol bisphosphate to form inositol trisphosphate and diacylglycerol5. The released inositol trisphosphate could function as a second messenger to mobilize Ca2+ from an intracellular store which in turn stimulates exocytosis5.
Functions
Caerulein produces several behavioral effects in mammals such as inhibition of food and water intake, changes in mood, analgesia, sedation and antipsychotic effects6. Caerulein also stimulates the secretion of pancreatic, bile and gastric juices1.
References
1. Anastasi A, Erspamer V & Endean R (1967). Isolation and structure of caerulein, an active decapeptide from the skin of Hyla caerulea. Experientia, 23, 699-700.
2. Anastasi A, Erspamer V & Endean R (1968). Isolation and amino acid sequence of caerulein, the active decapeptide of the skin of Hyla caerulea. Arch. Biochem. Biophys., 125, 57-68.
3. Caro GD, Endean R, Erspamer V and Roseghini M (1968). Occurrence of Caerulein in extracts of the skin of Hyla caerulia and other Australian hylids. Br. J. Pharmac. Chemother, 33, 48-58.
4. Book: Handbook of Biologically active peptides by Abba J Kastin, Pg 285.
5. Roberto B, Tullio P and Claes BW (1986). Caerulein and carbamoylcholine stimulate pancreatic amylase release at resting cytosolic free Ca2+. Biochem. J., 235, 139-143.
6. Zetler G (1985). Caerulein and its analogues: neuropharmacological properties. Peptides, 6, Suppl 3, 33-46.
| DOI | 名称 | |
|---|---|---|
| 10.1023/a:1006988625831 | Induction of permeability transition in pancreatic mitochondria by cerulein in rats | 下载 |
| 10.1371/journal.pone.0089114 | Bone morphogenetic protein signaling protects against cerulein-induced pancreatic fibrosis | 下载 |
| 10.1111/jvim.12319 | Qualitative and quantitative contrast-enhanced ultrasonographic assessment of cerulein-induced acute pancreatitis in dogs | 下载 |
| 10.1016/j.bmc.2014.04.043 | Design, synthesis, and characterization of novel apigenin analogues that suppress pancreatic stellate cell proliferation in vitro and associated pancreatic fibrosis in vivo | 下载 |
| 10.1016/j.jss.2015.02.032 | Apigenin inhibits pancreatic stellate cell activity in pancreatitis | 下载 |
| 10.1371/journal.pone.0165485 | The MET Receptor Tyrosine Kinase Confers Repair of Murine Pancreatic Acinar Cells following Acute and Chronic Injury | 下载 |
| 10.1016/0196-9781(85)90348-1 | Caerulein and its analogues: neuropharmacological properties | 下载 |
| 10.5114/ceji.2016.58816 | An optimised mouse model of chronic pancreatitis with a combination of ethanol and cerulein | 下载 |





